In situ anomalous powder diffraction study
of cation distributions in bicationic zeolites
H. Palancher1,2,
J.L. Hodeau2, C. Pichon1, J.F. Bérar2,3, J. Lynch1,
B. Rebours1 and J. Rodriguez-Carvajal4
1 Lab. de cristallographie, CNRS
BP166X 38042 Grenoble, France.
2 Institut Français du Pétrole, BP3 69390 Vernaison, France.
3 French CRG D2AM, ESRF, BP220 38043
Grenoble, France.
4 Lab. Léon Brillouin, CEA/Saclay, 91191 Gif/Yvette, France.
Adsorption
properties of molecular sieves such as X, Y or A zeolites are widely used in
industrial processes (in particular for separation and purification of
hydrocarbon isomers). Adsorption selectivity of these materials depends highly
on the type, the number and the location of cations in the structure where they
compensate the negative charge of the framework. The study of the cation
distributions on each site, under adsorption conditions close to their
industrial use, is of great interest in the search of better molecular sieves which
could be bicationic zeolites. In this work two methodological aspects will be
presented: first the in situ instrumentation and second the advances in
anomalous powder diffraction data analysis (required for cation distributions determination
in bicationic zeolites) illustrated by the study of CaSrX and SrRbX zeolitic
samples.
A cell has been
especially designed for the X-ray diffraction (XRD) study of powders under gas
or liquid flow, at various temperatures and pressures [1, 2]. It mainly
consists of:
- a reactor whose geometry enables
fix bed flow,
- a miniaturised cylindrical furnace
ensuring an extremely low thermal gradient (smaller than 1°C over a 6 mm zone
along the capillary at 250°C).
The limited
size of this cell has enabled its installation on a synchrotron radiation
beamline (D2AM at the ESRF). Thanks to this set-up, reproducible and high
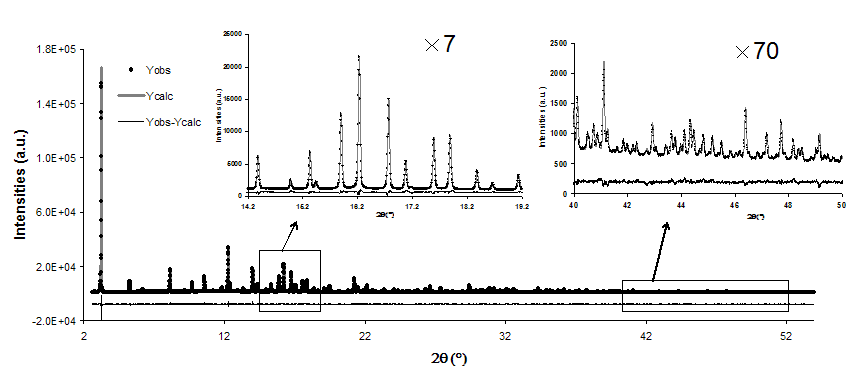
quality diffraction data can be measured (cf. figure I).
|
|
|
Figure I: Calculated and measured powder diffraction pattern
on water saturated CaSrX (E=15.192keV). |
In the
faujasite structure, cations occupy known sites. In bicationic zeolites, two
different cations are located in very close crystallographic positions. If
presence of both Sr++ and Rb+ cations on site II in
dehydrated SrRbX can be suggested from electron density map (figure II-B), this
technique appears inefficient when trying to determine Sr++ and Ca++
cation locations in dehydrated SrCaX: since electronic densities due to Ca++
coincide with those of Sr++, no site degeneracy can be observed. This
kind of problem can be encountered in certain monocationic hydrated zeolites
since water molecules can be adsorbed near cationic sites.
|
A-
|
B- d-SrRbX
|
C- d-SrCaX
|
|
4277.2 4001.3 3725.3 3449.4 3173.4 2897.5 2621.5 2345.6 2070.0 1793.7 1517.7 1241.8 965.8 689.9 413.9 138.0 -138.0 |
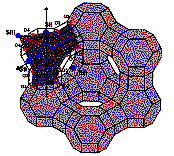
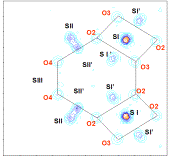
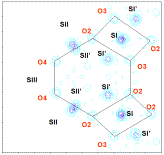
Figure
II: Study of
degeneracy of cation site (for example site II) on electronic density maps calculated
with Fourier transforms in the P plane (represented in dark grey in the
faujasite structure) (A-) in two dehydrated samples SrRbX (B-) and CaSrX (C-).
Anomalous
effect could be used to distinguish these species. This phenomenon leads to
strong variations with energy of the atomic scattering factor of an element
under an X-ray beam of energy close to one of its absorption edges. However
multi-wavelength measurements require new synchrotron sources to be performed
in good conditions. Diffraction patterns have been collected over a wide
angular range (sinq/l=0.57Å-1 at least) at
15192 eV (about 900 eV under strontium K absorption edge (EK(Sr)-900eV))
and at 16096eV (EK(Sr)-10eV) on SrCaX. A methodology for anomalous powder
diffraction data analysis has been established including simultaneous
refinement of all diffraction patterns using Fullprof software package [3]. Its
efficiency has been validated by the determination Sr++ and Rb+
cation distributions in SrRbX sample [4, 5]. This study was a particularly
difficult case for X-ray diffraction since Rb+ and Sr++
have the same scattering power (ZRb+= ZSr++=35e.u.). Note that, as Rb and Sr have similar neutron
scattering lengths (bRb≈bSr≈0.7.10-12cm-1),
neutron diffraction will not help us in this case.
CaSrX sample
has been characterised at two hydration levels: water saturated (measurement ex
situ at 20°C) and highly dehydrated (measurement in situ under dry
nitrogen flow at 250°C). Results of the refinements show the expected strong
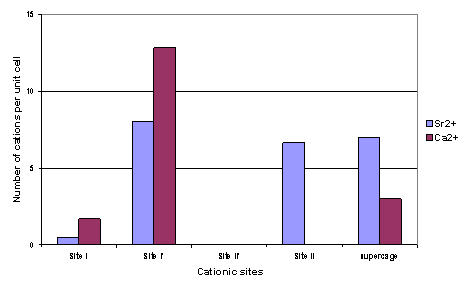
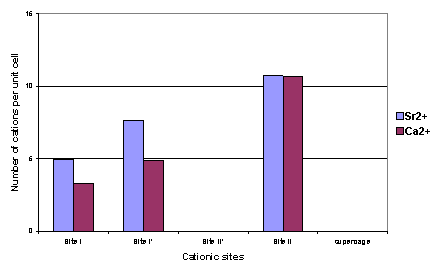
cation motions with water molecules loss [2] (figure III). If the very close distributions of Sr++
and Ca++ cations in the dehydrated sample could have been predicted
from chemical considerations (close cationic radii and same electric charge),
the very different behaviour of these cations in water saturated case
underlines the complexity of bicationic zeolites. Importance of accurate
measurements on these systems is demonstrated.
|
h -SrCaX
|
d- SrCaX
|
Figure III: Sr++ and Ca++
cation distributions in SrCaX sample at two hydration levels: water saturated
(h-SrCaX) and highly dehydrated (d-SrCaX).
1. Palancher, H., Pichon, C., Prévot, S., Conan,
G., Hodeau, J.L and Berar, J.F. (2003) Patent
03/07 641.
2. Palancher, H., Pichon, C., Hodeau, J.L.,
Lynch, J., Rebours, B., Berar, J.F. et al. (2004) submitted to JAC.
3. Rodriguez-Carvajal, J. (2002). Fullprof
version 2.10 LLB, CEA/Saclay, France,
http://www-llb.cea.fr/fullweb/poudres.htm.
4. Hodeau, J.L., Nassif, V., Palancher, H., Berar, J.F., Dooryhee, J.F., Carbonio,
E. et al. (2003) ECM Durban (South Africa).
5. Palancher, H., Hodeau, J.L., Pichon, C., J. Lynch,
Berar, J.F., Rebours, B. and Rodriguez-Carvajal, J. (2004) submitted.