The blue mineral pigment aerinite studied at high temperature with laboratory powder diffraction data
Jordi Rius1, Xavier Alcobé2
1Inst. Ciència de Materials de Barcelona (CSIC), Campus UAB, 08193 Bellaterra, Catalunya, Spain.
2Serveis Cientificotècnics, Univ. de Barc., Lluís Solé i Sabarís 1, 08028 Barcelona, Catalunya, Spain.
The structure of aerinite, a blue fibrous silicate mineral associated with the alteration of ophitic rocks in the southern Pyrenees has been recently determined by applying the direct methods modulus sum function to synchrotron powder diffraction data [1]. This mineral is the blue pigment commonly used in most Catalan romanic paintings between the XIV-XV centuries. The unit cell dimensions of aerinite are a=b=16.8820(9), c=5.2251(3) Å, the space group is P3c1 and the approximate structural formula is Ca5(Fe3+, Fe2+2,Al)(Al5 Mg)[Si12O36(OH)12H] with Z=1. The crystal structure of aerinite can be best understood by introducing cylindrical basic building units consisting on three pyroxene chains pointing inwards to accomodate tri- and divalent cations at the centre of the resulting face-sharing octahedra. The mean cation-oxygen bond length is 2.04 Å and the intercationic distance is 2.61Å. Out of the three symmetry-independent three-fold rotation axes in the unit cell, two are occupied by such cylindrical units and the third by CO3 groups. Consequently, each unit is surrounded by three similar ones which are, however, shifted by 0.93 Å along c. Between such units, i.e. tangential to both cylindrical envelopes, a four-row wide slab of a brucite layer is found. The tow inner octahedra are predominantly filled with Al and Mg atoms, the two outer with Ca and Na. The internal O atoms of the brucite-like layers are hydroxil groups, the intermediate are unshared basal O atoms of the neighbouring pyroxene chains, while the external ones are water molecules forming relatively strong H-bridges with the partially disordered CO3 groups (Figure 1).
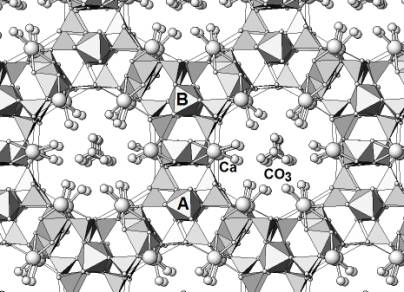
Figure 1
One peculiar property of aerinite that has been confirmed by infrared spectroscopy and TG measurements, is the loss of the CO3 groups when heated at 300ºC. When cooled down, however, aerinite slowly absorbs CO2 from the atmorsphere and, at the end, the resulting structure is identical to the initial one. This zeolitic behaviour necessarily involves a rearrangement of the coordination polyhedra. To study this reversible transformation, the same sample previously measured at the synchrotron was measured again at 25ºC and 300ºC on a laboratory X-ray powder diffractometer. On the basis of these two patterns, the mechanism that helps to stabilise the high temperature form of aerinite could be successfully derived from the corresponding Rietveld refinements. It has been found that both low and high temperature forms have the same non centrosymmetrical space group P3c1. The crystal structure of the high temperature form will be described in detail.
Acknowledgement
This
study was supported by the Ministerio de Ciencia y Tecnología, Project
Number:MAT2002-02808.
[1] J.Rius, E.Elkaim, X. Torrelles, Eur.J.Mineral. 16 (2004) 127-134.