THE KEY DTERMINANT IN GAS-GANGRENE: A NOVEL MEMBER OF THE C2 DOMAIN FOLD FAMILY?
C. E. Naylor1,
J. T. Eaton1, A. Howells2,
N. Justin1, D. Moss1,
R. Titball2 and A. K. Basak1
1Dept of
Crystallography, Birkbeck College, Malet Street, England, WC1E
7HX
2CBD, Porton Down, England.
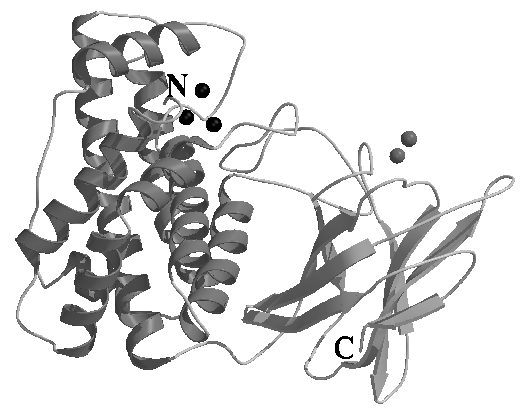
C. perfringens a-toxin is the key virulence determinant in gas-gangrene disease, and has also been implicated in a number of other diseases of man and animals. The toxin is a 370 amino acid zinc metalloenzyme of 43 kDa molecular weight, which has phospholipase C, sphingomylinase and haemolytic activities. In the presence of calcium ions, a-toxin can cleave membrane packed phospholipid. Here, we present the structure of the C. perfringens a-toxin, strain CER89L43. It is a 2 domain structure. The N-terminal, catalyti,c domain (residues 1-246) shows an anticipated structural homology to the non-toxic B. cereus phospholipase C. Both proteins contain 3 bound zinc ions: the residues that ligate the metal ions are completely conserved between the two proteins. There are a number of structural differences between a-toxin and the B. cereus enzyme, however, and these will be described in the talk. The C-terminal domain (residues 255-370) is essential for membrane binding, it shows a strong, unexpected structural analogy to the eukaryotic calcium binding C2 domain fold. We believe this is the first example of such a domain in prokaryotes. The C2 domain is normally found acting as a phospholipid and/or calcium-binding domain in intracellular second messenger proteins, and, interestingly these pathways are perturbed in cells treated with a-toxin. This homology has been useful in defining a possible model for membrane binding, which, when used in comparison with other, structurally analogous, but non-toxic, phospholipases, has illuminated the structural features important in a-toxin for toxicity.

Secondary Structure Cartoon of a-toxin. Zinc ions are shown as black spheres, possible calcium binding sites are highlighted as grey spheres.