UNDERSTANDING IgE ALLERGEN EPITOPES: COMPLEX FORMATION BETWEEN A Fab FRAGMENT OF A MONOCLONAL MURINE IgG ANTIBODY AND THE MAJOR ALLERGEN FROM BIRCH POLLEN bet v 1
Michael Gajhede1. Osman Mirza1, Henrik Ipsen2, Jørgen N. Larsen and Michael D. Spangfort2
1Protein Structure Group, Department
of Chemistry, University of Copenhagen, Universitetsparken 5,
DK-2100, Copenhagen, Denmark
2ALK-ABELLO Group, Bøge Alle 10-12,
DK-2970 Hørsholm, Denmark

The prevalence of allergic disorders has steadily increased in the Western world during the last decade and atopic individuals often show allergic reactions in response to protein allergens derived from pollens of trees and grasses, house dust and storage mites, animal dander and moulds. The type I immune response is characterised by the presence of allergen specific serum IgE antibodies produced as a result of B lymphocyte switching from IgG to IgE antibody production induced by the Th2 cytokine profile of allergen specific CD4+ T lymphocytes. Allergen specific serum IgE binds to the IgE high-affinity receptor, FceRI, localised on the surface of tissue mast cells and circulating basophils and clinical symptoms of allergy, i.e rhinitis, rhinoconjuctivitis ansd asthma, is a direct result of the cross-linking of receptor-bound IgE molecules and aggregation of FceRI receptors by a multivalent allergen to which previous sensitisation has occurred. Antigens must therefore have at least two B cell epitopes recognised by specific serum IgE in order to function as allergens.
The major source of tree pollen allergic sensitisation in the temperat climate zone consist of pollens from the Fagales.order of which birch is the clinically most important. Over 90% of all birch pollen allergic patients show specific serum IgE reactivity towards the 17.5 kDa major allergen Bet v 1 illustrating its importance in tree pollen allergy.
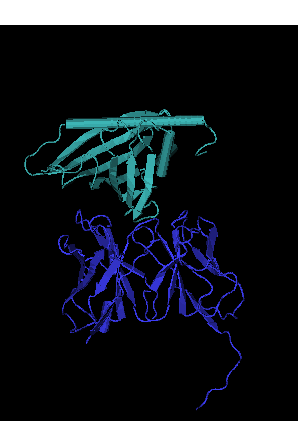
The structural basis for tree pollen allergic cross-reactivity was reported by Gajhede et al in 1996 1 where three patches on the molecular surface of Bet v 1 could be identified to be structurally similar for all the known major tree pollen allergens. Structure determination of allergens complexed to Fab fragments derived from murine monoclonal antibodies raised against naturally occuring allergens provides a more direct route for quantitative and qualitative studies of allergic B cell epitopes.
Here we report the crystal structures of birch pollen allergen
Bet v 1 complexed to Fab´derived from the murine monoclonal IgG
antibody BV16 to a resolution of 3.2 Å as well as that of a
genetically enginered Bet v 1 mutant (D45S) lacking reactivity
towards the BV16 antibody (resolution 3 Å).