THE CRYSTAL STRUCTURE OF
KB5-C20 scFv TCR / Désiré-1 Fab COMPLEX:
the first clonotype - anticlonotype
complex structure and a new topology for the Vb domains.
Housset D1, Mazza G2, Grégoire C2, Piras C2, Malissen B2 and Fontecilla-Camps J-C1
1Laboratoire de
Cristallographie et Cristallogénese des Protéines, Institut de
Biologie Structurale J.-P. Ebel, CEA-CNRS, 41, avenue des
Martyrs, F-38027 Grenoble cedex 1, France.
2Centre
díImmunologie de Marseille-Luminy, INSERM-CNRS, case 906,
F-13288 Marseille cedex 9, France
Keywords: crystal structure, immunology,
T-cell receptor, anticlonotype
There are two main classes of T lymphocytes: the helper T cells that will activate B cells or macrophages and the cytotoxic T cells that will kill infected cells. A cytotoxic T cell clone will recognise a specific peptide antigen (8-9 aminoacids) presented by a class I MHC (major histocompatibility complex) molecule at the surface of an infected cell. This recognition is accomplished by a multy-subunit transmembrane complex: the T cell receptor (TCR) / CD3 complex. The ab TCR is a clone specific immunoglobin-like heterodimer non covalently associated with an invariant polypeptide (CD3) responsible for the coupling with the intracellular signalling pathway.
The TCR a and b chains each consists of a N-terminal variable region and a C-terminal constant region, structurally related to light and heavy chains of immunoglobulins (Ig). The antigen binding site is formed by four hypervariable loops (CDR1, 2, and 3 for complementarity determining regions as in Igs, and HV4 specific to TCRs) on both Va and Vb. Since 1995, several crystallographic studies have revealed the structural specificities of ab TCR [1-6] as well as its mode of interaction with the peptide/MHC complex [3,4].
We rencently solved the crystal structure of the complex between a murine scFv TCR, KB5-C20, and a monoclonal anticlonotypic Fab, Désiré-1[6]. The first structure of a complex between an anticlonotypic Fab and its cognates TCR has shown a mode of interaction very different from that of idiotope-antiidiotope complexes, despite the structural similarities between TCRs and Igs. Since the Fab recognises CDRs of both Va and Vb, it is a true anti-clonotype. However, it does not mimic the MHC/peptide de complex, even if the bivalent Désiré-1 antibody activates the T-cells expressing KB5-C20 TCR.
The KB5-C20 TCR Vb is the first known Vb domains for which
the cíí b-strand is switched from the inner to the outer b-sheet, as in all
known Va
domains. Structure based TCR sequence alignement revealed that
other Vb
domains should present such a switch. Both Va and Vb CDR2 participate in the
binding to the MHC molecule, thus, the significance of the switch
on the CDR2 conformation will be discussed.
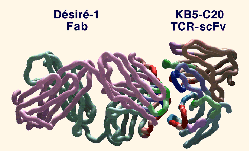
| Overall view of the complex between the KB5-C20 TCR scFv and DEsirE-1 Fab. The a- carbon backbone is represented as a tube. Framework residues are depicted in brown for Va, cyan for Vb, mauve for VL and forest-green for VH. CDRs are depicted in green for CDR1, red for CDR2, blue for CDR3, and purple for HV4. The residues that are in contact in the complex are highlighted by fully saturated colors. |  |
[1] Bentley, G.A., Boulot, G., Karjalainen, K.,
Mariuzza, R.A. (1995). Science 267, 1984-1987.
[2] Fields, B.A., Ober, B., Malchiodi, E.L.,
Lebedeva, M.I.,Braden, B.C., Ysern, X., Kim, J.-K., Shao, X.,
Ward, E.S., Mariuzza, R.A. (1995). Science, 270,
1821-1824.
[3] Garcia, K.C., Degano, M., Stanfield, R.L.,
Brunmark, A., Jackson, M.R., Peterson, P.A., Teyton, L., Wilson,
I.A. (1996). Science, 274, 209-219.
[4] Garboczi, D.N., Ghosh, P., Utz, U., Fan, Q.R.,
Biddison, W.E. and Wiley, D. (1996). Nature, 384,
134-141.
[5] Wang, J., Lim, K., Smolyar,
A., Teng, M., Liu, J., Tse, A.G.D., Liu, J., Hussey, R.E.,
Chishti, Y., Thomson, C.T., Sweet, R.M., Nathenson, S.G., Chang,
H-C., Sacchettini, J.C. and Reinherz, E.L. (1998). EMBO J.
17, 10-26.
[7] Housset D, Mazza G, Piras D, Malissen G, Fontecilla-Camps JC.
(1997). EMBO J., 16, 4205-4216.