THE CRYSTAL STRUCTURE OF THE SERINE/THREONINE KINASE DOMAIN OF THE GIANT MUSCLE PROTEIN TITIN REVEALS UNIQUE FEATURES FOR TIGHT REGULATION AND ATP BINDING.
Olga Mayans1, Mathias Gautel2 and Matthias Wilmanns1
1EMBL
Hamburg Outstation c/o DESY, Notkestrasse 85, D-22603
Hamburg,Germany
2EMBL Heidelberg, Meyerhofstrasse 1,
Postfach 10 22 09, D-69012 Heidelberg, Germany.
The giant muscle protein titin forms the third filament system in striated muscle cells of vertebrates. It has a molecular weight of about 3 MDa and a length of about 1 m, spanning half of the sarcomeric unit from the Z disc to the M line. Titin contributes to the muscle ultrastructure by acting as a molecular ruler during muscle assembly and is responsible for the muscle resting tension and elasticity, playing a role in muscle signal transduction (1). Titin is composed of 244 copies of immunoglobulin-like domains and fibronectin type-III domains arranged in a specific pattern (2), and one single serine/threonine kinase domain (TK) located in the P-zone, close to the C-terminus of the titin molecule.
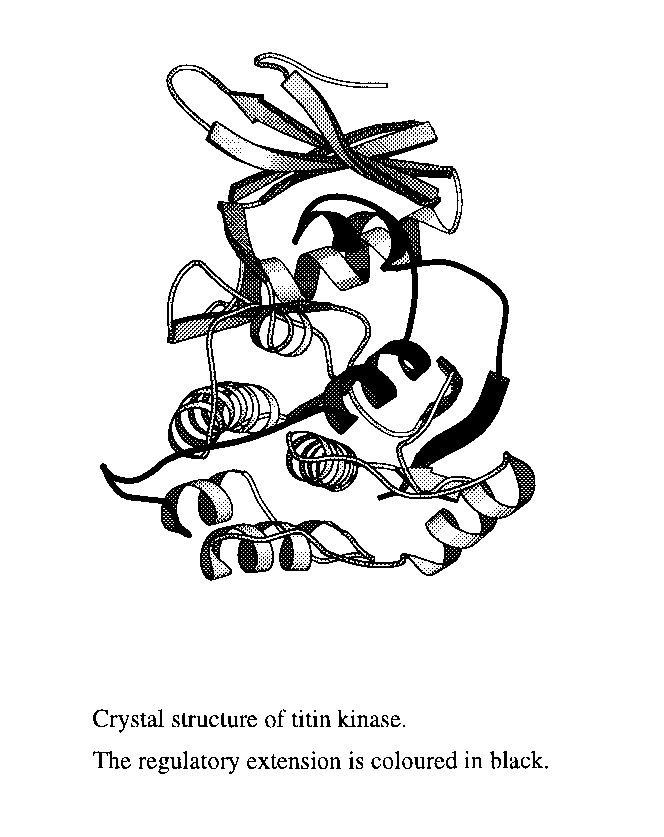
TK belongs to the family of myosin light chain kinases, all members of this being self-inhibited by a C-terminal extension of about 60 residues involved in intrasteric regulation (3). To date, x-ray structures for only one member of this family, the serine/threonine kinase of twitchin (TwK), have been reported (4,5). Twitchin is found in nematodes, where it plays a similar role to titin in ordering the muscle structure although its overall size is smaller, about 700 kDa. Despite the substantial sequence similarity in the catalytic domains of TwK and TK, these kinases have different substrate specificity, TwK acts on myosin light chain but TK does not. In addition, biochemical data suggest a different activation process for this two kinases, but the lack of sequence similarity in their regulatory parts does not allow to draw conclusions on the differences in their mechanisms of control based solely on the TwK structure.
To help elucidation of the role of TK in muscle signal transduction, we have determined the crystal structure of the autoinhibited form to 2.0 A resolution. Crystals were obtained from various crystallisation conditions but always as extremely thin plates, less than 5 m thick. Crystals belong to the P212121 space group with unit cell dimensions of a=78.61 A, b=89.77 A and c= 113.32 A and two molecules per asymmetric unit, the NCS copies being related by a two-fold axis parallel to a crystallographic screw axis. The structure has been solved by a combination of the molecular replacement and single-crystal averaging techniques and refined to a R-factor of 20.7% and a R-free of 24.8% for all reflections in the resolution range 40.0 to 2.0 A.
The structure has revealed that
the activity of TK is tightly regulated by a two-step activation
process, achieved by a combination of features not previously
observed. The active site of this kinase presents unusual amino
acid substitutions affecting both the ATP binding site and
catalytic residues. Conclusions on substrate specificity and
activation have been drawn and biochemically tested.
