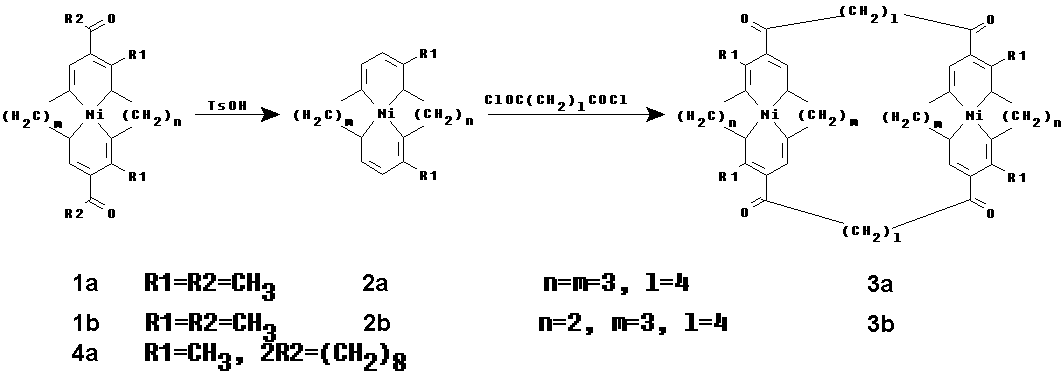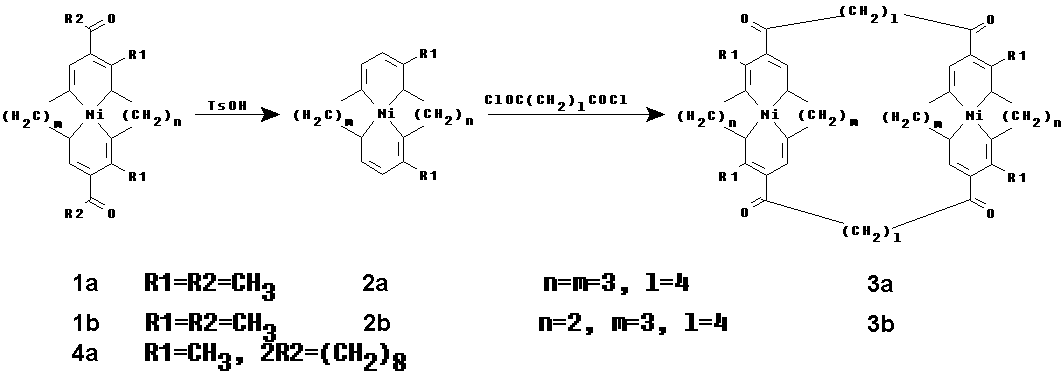
SYNTHESIS, STRUCTURE AND UNUSUAL VOLTAMMETRIC BEHAVIOR OF FACE-TO-FACE BISMACROCYCLIC Ni(II) RECEPTORS IN COMPLEXES WITH SMALL ORGANIC GUESTS
A. Olszewska1, R. Bilewicz1, A. Wieckowska 1, B. Korybut-Daszkiewicz2, K.WoYniak1
1Department
of Chemistry, University of Warsaw, ul. Pasteura 1,02-093
Warszawa, Poland.
2Institute of Organic
Chemistry, Polish Acadamy of Sciences, ul. Kasprzaka 44/52,
01-224 Warszawa, Poland.
The purpose of this study was to design macropolycyclic molecules functioning as heterotopic co-receptors allowing one to bring together, within the same supramolecular structure, transition metal ions and organic substrates. The enforced proximity of the metal centres is interesting from the view-point of modelling of the multimetallic active centers of metalloproteins. The main interest, from the electrochemical point of view, is connected with the possibility of producing complementary 2e processes and multielectron pathways for the catalytic reduction of O2, CO2 or N2.
Our interest in these compounds derives from the fact that the rigid components of those molecules lead to the formation of a well-defined empty cavity, that might accommodate other molecules of appropriate size. This would be observed in the structure of the complexes in the solid state, but also may be detected voltammetrically in solutions. Such ligands are build of two macrocyclic metal ion coordination sites linked with two hydrocarbon chains. Well-known macrocyclic Schiff bases (Scheme 1; compounds 1a and 1b) were chosen as the metal coordinating sites. Nickel(II) complexes 1a and 1b after deacetylation yield very reactive neutral species 2a and 2b which can be easily acylated with different acyl chlorides. The shape of the hydrophobic cavity can be modified by changing the macrocycle size and/or the length of the hydrocarbon linkers. The details of synthesis, X-ray and voltammetric studies will be presented.
Scheme 1