STEROID RECEPTOR BINDING: A COMPARISON OF AN EMPIRICAL MODEL AND THE OBSERVED STRUCTURE
William L. Duax and Jane F. Griffin
Hauptman-Woodward Medical Research Institute, Inc. 73 High Street, Buffalo, New York 14203 USA duax@hwi.buffalo.edu
The characteristic responses of steroidal hormones require their binding to specific receptor proteins in target tissue. The existence of antagonists that compete for the steroid binding site of the receptor with high affinity demonstrates that the phenomena of binding and activity are at least partially independent. On the basis of examination of the X-ray crystal structures of compounds having high affinity for estrogen, progestin, and corticoid receptors, we proposed that receptor binding is primarily the result of a tight association between the receptor and the steroidal A ring. We postulated that agonists and antagonists that compete for a common site on a steroid hormone receptor must have A rings of identical or closely comparable composition that would account for their binding to a common site and differences in the D ring region such that features critical to induction of hormone response are present on agonists while antagonists should lack these required features andlor have other structural features that interfere with subsequent receptor functions essential to activity [1]. Our 'A-ring binding/D-ring acting' model for progestins and for estrogens, first proposed in 1978, was viewed with skepticism because the prevailing theory was that the high affinity of steroids for the receptor suggested a close lock and key fit over the entire steroid surface so that all potential hydrogen bonds and van der Waals contacts could contribute to binding.
All of these predictions are confirmed with the recently determined crystal structures of the estrogen receptor binding domain with agonist (estradiol) and antagonist (relaxifene) bound [2].
We speculated in 1980 that the D rings of estrogens, progestins, and corticoids might control activity by:
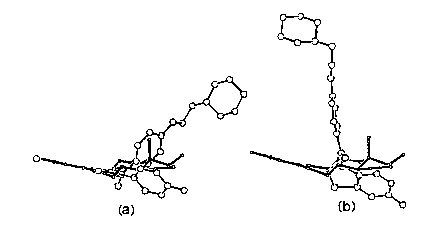
The comparison of the estrogen receptor binding domain complex revealed by X-ray structure determinations indicates that the first mechanism certainly applies. It may be too soon to eliminate proposals 2 and 3 as additional hormone functions. In an effort to fine tune the empirical model so that it could more effectively be used in drug design, we compared estrogen agonists and antagonists [3]. We considered two possible ways in which the antiestrogen LY 156758 (Relaxifene) would occupy the estrogen binding site with respect to the fit of estradiol, differing as to which of two phenolic rings in relaxifene would mimic the estradiol A ring (Figure 1).

The recent X-ray structure determination of the complexes of estradiol and LY156758 with the estrogen binding domain of the receptor indicates that the predicted it illustrated in Figure la was remarkably accurate.
Research supported by NIH Grant No. DK26546.