HALIDE SILICIDES OF RARE EARTH METALS - Sin UNITS WITH n = 6, 12, 14, 22 AND INFINITE CHAINS
H. Mattausch, O. Oeckler, A. Simon
Max-Planck-Institut für Festkörperforschung, D-70569 Stuttgart, Deutschland
Keywords: Rare Earth Metals, Halides, Silicides, Metal-Metal Bonding, Sin Rings.
Numerous metal-rich halides REXnA (n<=2) of Rare Earth metals RE with interstitials A = H, B, C, N, O could be prepared. Due to the electropositive character of RE the interstitials occur as anions, and in the case of carbon C26- and C24- are found besides discrete C4- ions. B: C, C-B-C, C-B-B-C and (C)2-B-B-(C)2 units are characteristic fragmerts of the structures of the halides of Rare Earth metal boride carbides. So far, the only compounds with silicon as interstitials are Gd4I5Si and Gd3I3Si. Single Si atoms center Gd6 octahedra similar to carbon in the corresponding carbide halides.
Here we report about Rare Earth metal halide silicides with larger Sin entities: Si6, Si12, Si14 and Si22 rings besides Si-Si zig-zag chains. The compounds are prepared in quantitative yield by annealing stoichiometric mixtures of REX3, RE and Si in closed Ta capsules at ~l000oC for several days, and they form needles or laths with metallic lustre. The compounds are extremely sensitive to moisture and ignite explosively in contact with water. Preparative and crystallographic détails are summarized in table l.
tab.l: Halide Silicides of RE Metals; preparation temperature T, space group and lattice parameters, () standard deviation, Sin entity.
| compound | T(oC) | S.G. | a | b | c | b | Sin |
|---|---|---|---|---|---|---|---|
| LaSiI | 1000 | P3_m1 | 4.2193(2) | - | 11.794(1) | - | Si6 |
| CeSiI | 1000 | P3_m1 | 4.1799(4) | - | 11.693(1) | - | Si6 |
| PrSiI | 950 | P3_m1 | 4.1635(4) | - | 11.619(1) | - | Si6 |
| La6Si7Br3 | 950 | Pmmm | 16.868(2) | 4.130(1) | 11.836(2) | - | Si12 |
| La4Si4I3 | 950 | C2/m | 24.359(2) | 4.243(1) | 12.572(2) | 97.55(1) | Si6,Si14 |
| Ce4Si4I3 | 900 | C2/m | 24.126(2) | 4.207(1) | 12.447(1) | 97.57(1) | Si6,Si14 |
| La5Si5I3 | 1100 | C2/m | 24.039(2) | 4.257(1) | 15.722(2) | 110.40(1) | Si22 |
| La3Si3Cl2 | 950 | C2/m | 18.081(2) | 4.236(1) | 10.618(1) | 97.94(1) | 1 INFINITY |
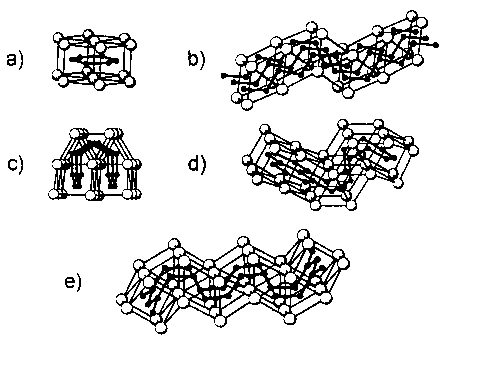
In the crystal structures of these compounds the Si atoms exhibit trigonal prismatic coordination by the RE atoms. Fusing the RE prisms parallel (p) or orthogonal (o) to the directions of the quasi 3-fold axis leads to different kinds of Sin units (compare fig.l).
fig.l: Characteristic Building Units of Halide Silicides of RE Metals.
a.) Si6 rings: CeSiI b.) Si zig-zag-chains: La3Si3Cl2 c.) Si12 rings: La4Si7Br3 d.) Si6, Si14 rings: La4Si4I3 e.) Si22 rings: La5Si5I3

In the structure of CeSiI prisms with only (p) orientation form layers, and the occupation of the centers results in sheets of Si6 rings. Cis- trans connection in (p) orientation leads to undulated layers of the RE prisms with zig-zag-chains of the Si &127atoms parallel [00] for La3Si3Cl2. In contrast to these two compounds the trigonal prisms in La4Si4I3 are fused both in the (p) and (o) mode. Double strings of Las prisms (o) are alternatingly condensed with prisms (p) to form wavy layers with ribbons of trans condensed Si6 rings. The occupation of the centers of prisms (o) by Si atoms and the connection with the Si6 rings leads to the hitherto unknown Si14 rings. A similar arrangement of wavy layers is found in the structures of La6Si7Br3 and of La5Si5I3 with sheets of condensed Si12 and Si22 rings, respectively.
The halogen atoms surround the RE-Si-RE layers on both side above and below the trigónal faces and connect the sheets to each other.
The description of the compounds within the Zintl - Klemm - formalism corresponds to Ce3+Si(3b)l-I-ˇ le-, La3+3Si(2b)2-3Cl-2ˇle-, La3+4Si(3b)l-2Si(2b)2-2I-3ˇ3e-, La3+5Si(3b)l-lSi(2b)2-4I-3ˇ3e- and La3+6Si(3b)l-2Si(2b)2-5Br-3ˇ3e-, respectively. Either one or three electrons occupy bands with RE-RE bonding character. In agreement with the observed metallic conductivity as well as the results of band structure calculations these electrons are delocalized.