REVERSIBLE REDOX PROCESSES IN
HOMOGENEOUS PHASES
Bärbel Bodensohn, Martin Trömel
Institut für Anorganische Chemie,
Marie-Curie-Strasse 11, 60439 Frankfurt, Germany
bb@solid.anorg.chemie.uni-frankfurt.de
Keywords: lanthanoid lead oxides, mixed valent oxides, nonstoichiometric oxides, oxidation, reduction
Nonstoichiometric lanthanoid lead oxides LnxPb(II, IV)1 - x O1.5 + d (x » 0.3 - 0.9, - 0.2 < d < 0.2) with varying Pb(IV)/Pb(II) ratio are formed by thermal decomposition of the corresponding hydroxides [1], [2], [3]. By X-ray and neutron investigations of powder samples, their structure is determined as fluorite-type structure with defects in the oxygen lattice (space group Fm3m, four heavy atoms per cell). Lanthanoid and lead atoms are distributed statistically in the (4a) position, oxygen partially occupies the (8c) position. The disorder causes high displacement parameters of all atoms that increase with temperature. Bismutates and zirconates of this structural type are known to be fast ion conductors [4], [5], [6].
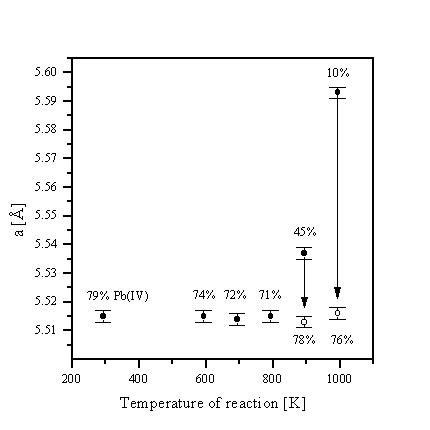
Increasing Pb content leads to a decrease of the lattice constant. A reduction of the lattice parameter a is also observed if the temperature of preparation is raised which raises the content of tetravalent lead.
According to X-ray powder
investigation and chemical analysis, the lanthanoid lead oxides
loose oxygen between 600 and 1000 K (1 h) under nitrogen by
reduction of tetravalent lead which is accompanied by an increase
of lattice constant. Re-oxidation by oxygen at 573 K (0.5 h)
restores the initial cell parameter and state of oxidation
(Figure 1). Both processes were also observed in situ by
high-temperature X-ray diffraction. 
Figure 1: La0.45Pb0.55Oy: Variation of lattice parameter a and Pb(IV) content due to reduction (·) and oxidation (o).