RB/CS-STANNIDES, -STANNATES AND -STANNID-STANNATES
Constantin Hoch, Caroline Röhr and Peter Zönnchen
Institut für Anorganische
und Analytische Chemie, Universität Freiburg, Albertstr. 21,
D-79104 Freiburg,
e-mail: caroline@ruby.chemie.uni-freiburg.de ; www: http://ruby.chemie.uni-freiburg.de
Keywords: Stannates,
Stannides, Zintl phases, Raman spectroscopy.
The reduction of oxides of main group elements M (M=tetreles, e.g. Sn) with elemental Rb and Cs (A) yields not only new border phases of the metal rich region of the ternary systems A-M-O, new stannates(II) on one hand and new binary intermetallic phases on the other hand, but also metal rich compounds that contain the anions of both types of compounds side by side.
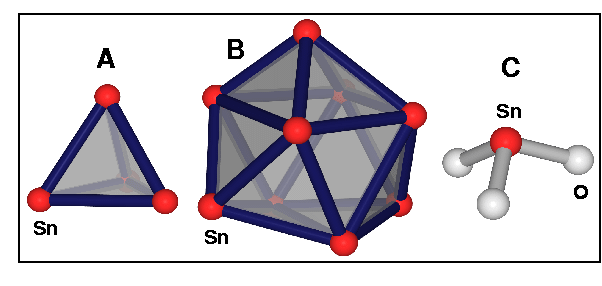
The binary Zintl phases of the KSn structure type [1] contain M44- tetrahedra which are isosteric to white phosphorus (Fig 1. A). The structures of NaPb [2], KPb [3], KSn [4] and CsPb [5] have already been refined from single crystal data. RbSn (tetragonal, I41/acd, Z=32, a= 1174.0 pm, c=1918.7 pm, R1=0.069) fits into that series showing the unusual trend of decreasing M-M distances with increasing atomic number of the alkaline metal.
From the series of binary intermetallics with composition AI12MIV17 only the disordered phases Na12Ge17 and K12Ge17 [6] were known from single crystal data. Some others were identified from vibrational spectroscopic data [6]. Rb12Sn17 (orthorhombic, P212121, a=1502.7 pm, b=1542.5 pm, c=2146.2 pm, Z=4, R1=0.113) is the first compound refined from single crystal data without any disorder of the cluster anions. The compound contains two different anions according to a decomposition Rb12Sn17 -> 12 Rb+ + 2 Sn44- + Sn94-. Besides the known P4-isosteric Zintl anion [Sn4]4- (Fig. 1 A) clusters [Sn9]4- (see also [6,7], Fig. 1 B) are present, which can be described - according to Wades rules - as 22 e- nido M10 clusters. The Sn-Sn distances in [Sn4]4- (290.2 - 295.1 pm) are comparable to those in RbSn (293.3-293.4 pm). The intra-cluster distances vary from 278.4 to 317.7 pm.
On the other hand, the K/Cs stannates(II) A4SnO3 [8,9] have also been described recently. Their crystal structures contain psi-tetrahedra [SnIIO3]4- (Fig. 1 C).
 |
 |
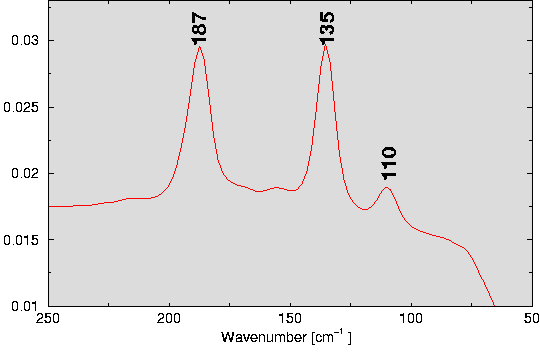
| Fig. 1: Anions in Rb/Cs stannides, stannates and stannid-stannates: A: [Sn4]4-; B: [Sn9]4-; C: [SnO3]4- | Fig. 2: [Sn4]4- vibrations in the Raman spectrum of Rb23.6Sn7.4O13.2. |
The reduction of SnO and Sn with elemental Rb (ratio Rb:Sn:O=12:5:3, Tmax=280 oC) yields dark red crystals of the composition Rb23.6Sn7.4O13.2 (monoclinic, P21/c, a=2174.2 pm, b=1134.0 pm, c=2373.6 pm, ß=116.1 o, R1=0.047). According to an ionic splitting Rb23.6Sn7.4O13.2 -> 23.6 Rb+ + 3 O2- + 3.4 SnO34- + Sn44- the structure exhibits the building blocks of stannates(II) (psi-tetrahedra [SnIIO3]4- comparable to those in Cs4SnO3; dSn-O = 199.8-204.6 pm, Fig. 1 C) and of the AM stannides (Zintl phases) ([Sn4]4- tetrahedra comparable to those in RbSn; dSn-Sn=291.0-292.3 pm, Fig. 1 A). Diffuse streaks in the diffraction pattern running parallel to a* at c*/2 can be explained on the basis of a disorder of one of the four crystallographically independet SnO34- and Rb+ resulting in the non-integer composition of the compound. Because of the higher transparency of the stannid-stannate, it was possible to record highly resolved Raman spectra of the Zintl anion [Sn4]4-, while for the pure AM phases only spectra of low resolution have been determined [10]. The band positions of the three Raman active modes (Gamma = A1(R) + E(R) + T2(R,IR) ) are in good agreement with those known from KSn and the compounds A12Sn17 [6]. The results of Sn Mößbauer spectroscopic studies (chemical shifts of Sn2+ in [SnO3]4- and Sn- in Sn44-) will be discussed.
1. E. Busmann, Z. Anorg. Allg. Chem.
313 (1961) 90-106.
2. C. Röhr, Habilitationsschrift,
TH Darmstadt (1996).
3. C. Röhr, Z. Naturforsch. 50b
(1995) 802-808.
4. I. F. Hewaidy, E.Busmann, W. Klemm, Z.
Anorg. Allg. Chem. 283 (1964) 283-288.
5. M. Somer, W. Carrillo-Cabrera, K.
Peters, H.G. von Schnering, VIth European Conference on Solid
State Chemistry, (Zürich) (1997) PA122.
6. H. G. von Schnering, M. Baitinger, U.
Bolle, W. Carrillo-Cabrera, J. Curda, Y. Grin, F. Heinemann, J.
Llanos, K. Peters, A. Schmeding, M. Somer, Z. Anorg. Allg.
Chem. 623 (1997) 1037-1039.
7. M. Somer, W. Carrillo-Cabrera, K.
Peters, E. M. Peters, H. G. von Schnering, VIth European
Conference on Solid State Chemistry, (Zürich) (1997) PA92.
8. C. Röhr, P. Zönnchen, Z. Anorg.
Allg. Chem. 624 (1998) 797-801.
9. C. Röhr, Z. Anorg. Allg. Chem.
621 (1995) 757-760.
10. G. Kliche, H. G. von Schnering, M.
Schwarz, Z. Anorg. Allg. Chem.608 (1992) 131-134.