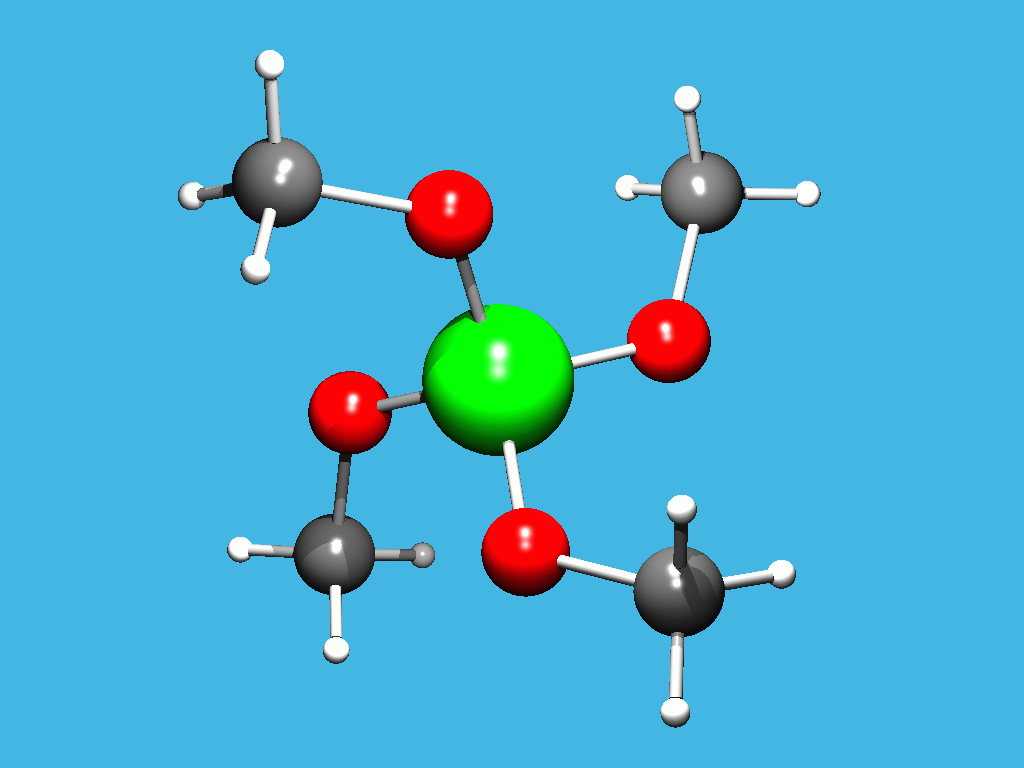
CRYSTAL STRUCTURE OF TETRAMETHOXYSILANE
Gerhard Müller1 and Martin Lutz2
1 Universität Konstanz, Fakultät
für Chemie, 78464 Konstanz, Germany
2 Bijvoet Center for Biomolecular
Research, Department of Crystal and Structural Chemistry, Utrecht
University, 3584 CH Utrecht, The Netherlands
Tetramethoxysilane is a simple derivative of ortho silicic acid, H4SiO4, which itself is inaccessible in the solid state. A first crystallographic study of tetramethoxysilane was performed in 1931 with Debye-Scherrer methods and a sophisticated low temperature device [1], because the melting point is -4oC. The structure was solved in the cubic space group P23 (a = 9.85(1) A) and the Si-O-C angle was considered to be exact 180o, because of the tetrahedral symmetry of the silicium atom.
We have now repeated the structure determination on a four circle diffractometer. A suitable single crystal was grown directly on the diffractometer with a modified zone melting process [2] and the data were collected at -90oC. We also found a cubic unit cell, but with a larger volume (a = 11.669(1) A). We could refine the structure in the non-centrosymmetric space group P4_3n with two independent molecules and the assumption of orientational disorder, which is not unusual with tetrahedral and spherical molecules in the plastically crystalline state. The silicium is located on a 4_ axis in the first molecule and on a threefold axis in the second molecule. Therefore in both molecules the carbon atoms can be treated without disorder model. Only the oxygen atoms and in the first molecule the hydrogen atoms are disordered.
Like other plastically crystalline compounds tetramethoxysilane undergoes a solid-solid phase transition when it is cooled further down. Measurements with Differential Scanning Calorimetry show transition temperatures at -110.2oC during cooling and -106.6oC during heating at 20K/min. Unfortunately we could not get a crystal structure of the low temperature phase.
