THE CRYSTAL STRUCTURE OF THE „451-PHASE"
Räth, S. and Schmahl, W. W.
Inst. f. Mineralogie, Petrologie u.
Geochemie, Univ. Tübingen, Germany.
e-mail: wwschmahl@uni-tuebingen.de
Keywords: 451-Phase, 3321-Phase, 231-Phase, BSCCO,
HTSC.
For the fabrication of 2223-HTSC wires or tapes, Pb-doped 2223 gives best results, due to promotion of crystal growth probably by a Pb-rich melt. The multiphase precursor powder for the PIT-process is usually reported to contain 2212, Ca2PbO4 and other minor phases. However, Majewski [1] and Jeremie [2] described the formation of a „451-phase", now recognized as Pb3,5Sr5,5Cu1,2O13,7-x, in their precursor powders, which had previously been overlooked or mistaken for Ca2PbO4.
The „451-phase" and its wide solid solution range was first described by Kitaguchi [3]. Reported compositions differ very much from each other: Kim et al. [4] determined by iodometric titration Sr4,8Pb3,2Cu1,6O11,76 and Sr4,8Pb3,2Cu0,7O12,3, Majewski supposes from EDX-measurements Pb4Sr5CuO10 and Jeremie assumes (Bi,Pb)3Sr3Ca2CuOx.
The structure and crystal chemistry of this substance (also
called „231" or „3321"-phase have hardly been the
subject of detailed examinations. Kim et al. proposed the
spacegroup P-62m on the base of single-crystal X-ray-data, but
their two copper-sites, which are just 0.9 apart, with site
occupancies of 0.197 or 0.134, respectively, are questionable and
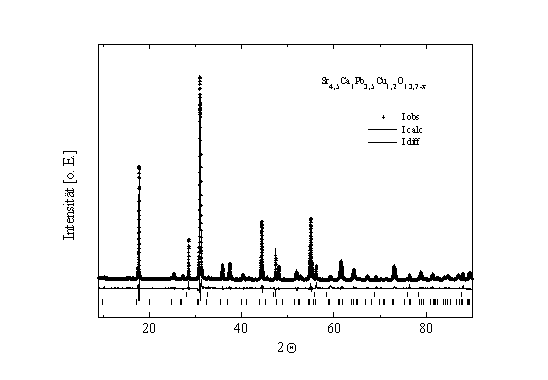
should be investigated closer. After Rietveld analysis of X-ray
data (Fig. 1), we suppose only one copper-site with full site
occupancy.

Fig. 1: Rietveld refinement plot of the sample
Sr4,5Ca1Pb3,5Cu1,2O13,7-x.
To determine the correct atomic position of Cu and the
preferential sites for Ca and Bi substitution in the
„451"-solid-solution, we examined three phase pure samples
of composition Sr5,5Pb3,5Cu1,2O13,7-x,
Sr3,5CaPb3,5Cu1,2O13,7-x and
Sr5,5Pb2,5BiCu1,2O13,7-x
by X-ray Rietveld refinement. The data were collected on a Huber
644 Guinier-diffractometer (Cu-K1-radiation, 40kV/20mA, 0.01°,
9-90° 2) with Si as an internal standard. Ca occupies the
Sr2-Position (3g), Bi the Pb-Position (3f) of Kim et al. [4].