SYNTHESIS AND CRYSTAL
STRUCTURE OF [Zn(CH3COO)2L2]·2CH3OH
(L = 3-AMINO-5-PHENYLPYRAZOLE)
Z.
K. Jacimovic1,
G. A. Bogdanovic2
, Z. D. Tomic2,
E. Z. Iveges3,
V. M. Leovac3
1Faculty of Metallurgy and Technology,
University of Montenegro,81000 Podgorica, Yugoslavia;
2Institute of Nuclear Sciences
"Vinca", 11001 Belgrade, Yugoslavia;
3Institute of Chemistry, Faculty of
Sciences, University of Novi Sad, 21000 Novi Sad, Yugoslavia
Keywords: Zinc(II)-complex; Ligands: acetato and
3-amino-5-phenylpyrazole; crystal structure
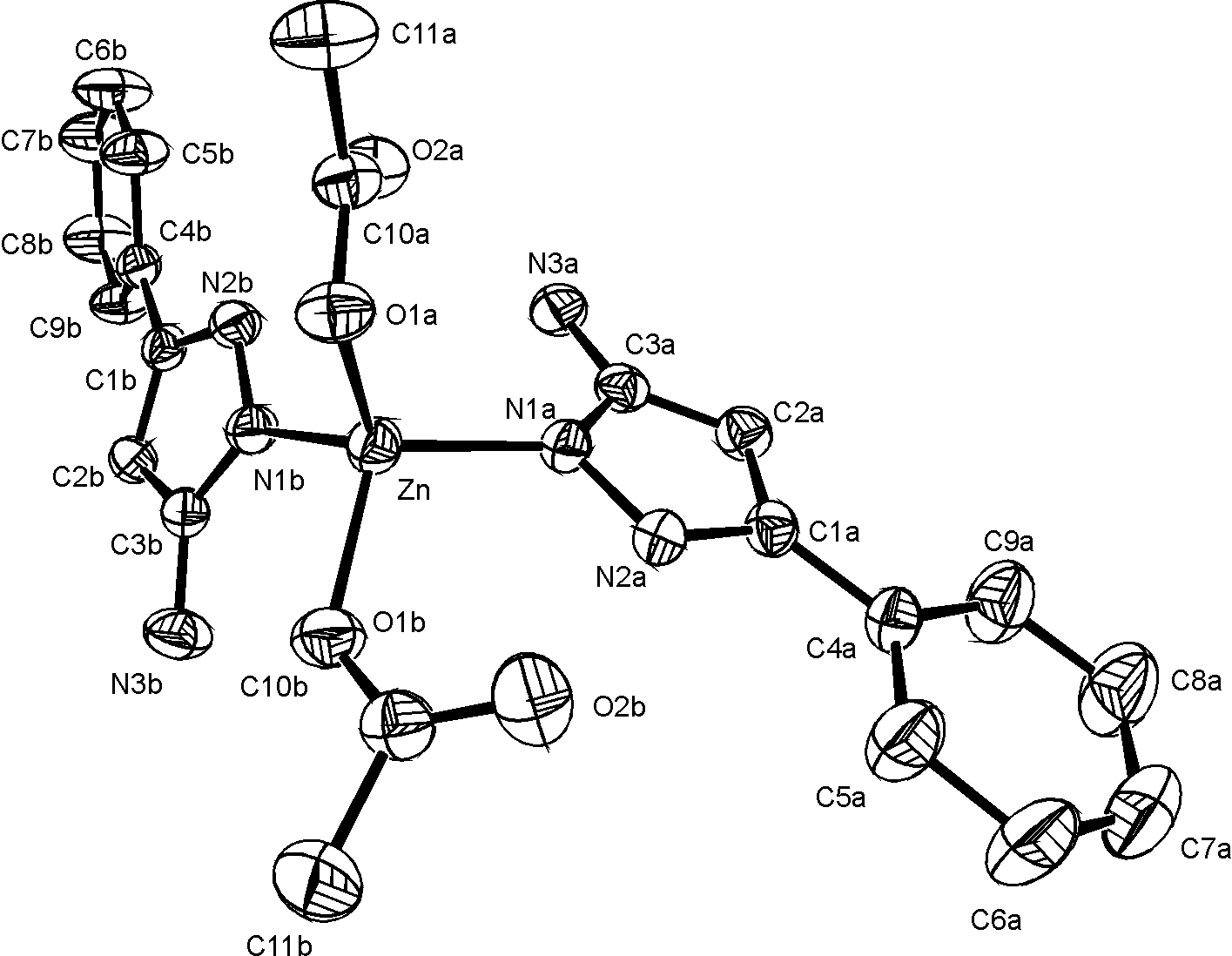
Monocrystals of the titled complex were obtained by crystallization from methanolic solution of Zn(CH3COO)2 · 2H2O and 3-amino-5-phenylpyrazole (L), (mole ratio 1:2).
The compound crystallizes with two solvent methanol molecule in the triclinic space group, P-1, with a=9.377(1) A, b=9.940(1) A, c=15.344(1) A, a =93.06(1)o, b =98.17(1)o, g =98.23(1)o, V=1397.2(2) A3, Z=2, Dc=1.33 g/cm3, l(MoK)=0.71073 A.
The crystal structure was solved by Patterson and difference electron density synthesis and refined by full-matrix least-squares calculations to a final R=4.54 and Rw=5.62 based on the 3753 observed reflections (I>3 s(I)).
Two pyridine nitrogen atoms from pyrazole derivative and two oxygens from acetato groups are coordinated to Zn in a distorted tetrahedral arrangement. The phenyl and amino- pyrazol rings in A and B ligands are both planar within the experimental accuracy. Dihedral angle between phenyl and amino-pyrazol planes are 5.6(2)° in ligand A and 27.4(2)° in ligand B.
