NONSTOICHIOMETRIC TITANATES:
COMPARISON BETWEEN XRD, NEUTRON DIFFRACTION, AND BOND VALENCE
METHODS
M.Yu.Avdeev1, G.V.Shilov2,
V.B.Nalbandyan1,
V.A.Volotchaev1, L.O.Atovmyan2,
A.I.Beskrovniy3, A.M.Balagurov3
1 Chemical Faculty, Rostov State
University, ul.Zorge 7, 344090 Rostov-on-Don, Russia,
E-mail: mavdeev@uic.rnd.runnet.ru
2 Institute of Chemical Physics, Russian
Academy of Science, Chernogolovka, Russia
3 Frank Laboratory of Neutron Physics, Joint
Institute for Nuclear Research, Dubna, Russia
Keywords: crystal structure, X-ray
diffraction, neutron diffraction, bond valence, nonstoichiometry
The structures listed in the table are solved and/or refined
using single crystal X-ray diffraction (X) and/or powder neutron
time-of-flight diffraction (N). All the phases, except No. 2 and
3, exhibit Na+ ion conduction and ion exchange
properties. The structural aspects of ion transport are
discussed. Interstitial sodium sites are found and refined, and
local cation arrangements are discussed in terms of Na+
- Na+ repulsion and transport mechanisms. The phases
No. 1, 3, 10 - 13 contain heterovalent component (e.g. Mg2+,
Zn2+, Sc3+, Fe3+, Cr3+)
distributed over several non-equivalent octahedral Ti4+ sites.
X-ray data of these crystals (or their isomorphs [1-5]) were
interpreted in terms of uniform distribution of these species.
However, bond valence calculations (B) and comparison of bond
lengths (based upon the same X-ray data) have shown a preference
of the larger M2+ or M3+ cations to the
specific sites. Neutron diffraction and re-examination of the
X-ray data usually confirm these results. The accuracy of the
bond valence method [6] for occupancy determination is close to
that of the neutron diffraction method. A comparison of the bond
valence schemes by Pyatenko [6] and by Brown [7] have shown the
former to be more appropriate.
No
|
Formula
|
Space group
|
a, A
|
b, A
|
c, A
|
b,
0
|
Method
|
1
|
Na5,4(Mg0,7Ti7,3)O18
|
C2/m
|
23,06
|
2,944
|
10,69
|
103.6
|
X, B, N
|
2
|
Na(Na0,3Li0,7)Ti3O7
|
Fmmm
|
16,537
|
5,755
|
11,208
|
|
X
|
3
|
Na(Na0,4Zn0,6)(Zn0,3Ti2,7)O7
|
Fmmm
|
16,675
|
5,725
|
11,258
|
|
X, B
|
4
|
(Na,Li)0,9(Li0,3Ti0,7)O2
|
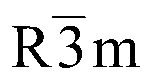
|
3,000
|
|
16,081
|
|
X
|
5
|
Na0,66(Li0,22Ti0,78)O2
|
P63/mmc
|
2,959
|
|
11,122
|
|
X, N
|
6
|
Na0,64(Ni0,32Ti0,68)O2
|
P63/mmc
|
2,960
|
|
11,187
|
|
N
|
7
|
Na0,72(Ni0,36Ti0,64)O2
|
P63/mmc
|
2,964
|
|
11,106
|
|
N
|
8
|
Na0,60(Cr0,60Ti0,40)O2
|
P63/mmc
|
2,929
|
|
11,212
|
|
N
|
9
|
Na0,50(Cr0,60Ti0,40)O2
|
P63/mmc
|
2,923
|
|
11,258
|
|
N
|
10
|
Na4,4(Fe0,4Ti4,6)O12
|
C2/m
|
26,518
|
2,949
|
6,323
|
95,81
|
B, N
|
11
|
Na9(ScTi10)O26
|
C2/m
|
37,559
|
2,979
|
9,443
|
93,66
|
B, N
|
12
|
Na5,8(Mg1,4Ti4,6)O13F
|
P2/m
|
15,814
|
2,964
|
6,291
|
89,84
|
N
|
13
|
Na0,8(Cr0,8Ti1,2)O4
|
Pnma
|
9,206
|
2,930
|
11,33
|
|
B, N
|
The work was supported by the Russian Foundation for Basic
Research grant No. 97-03-33807a.
- J.Akimoto, H.Takei. J. Solid State
Chem. 1989, v. 83, p.132.
- R.Werthmann, R.Hoppe. Z. anorg.
allgem. Chem. 1984, v.519, p.117.
- G.V.Shilov, L.O.Atovmyan. Crystallography
Reports, 1995, v.40, p.824 (in
Russian).
- O.S.Filipenko et al. Russian J. Inorg.
Chem. 1989, v.34, p.1100 (in
Russian).
- W.G.Mumme, A.F.Reid. Acta cryst.,
1968, v.B24, p.625.
- Yu.A.Pyatenko. Kristallografiya,
1972, v.17, p.773 (in Russian).
- I.D. Brown. Acta Cryst., 1985, v.B41,
p. 244.