 ,
,PART OF THE "THIRD COMPONENT" (Ln', M) IN CRYSTAL STRUCTURE FORMATION OF Ln(Ln',M)X (X=S, Se, Te) SYSTEM COMPOUNDS
Matveenko I.A., Kuz'micheva G.M.
M.V. Lomonosov State Academy of Fine
Chemical Technology, Moscow 117571, Russia
E-mail: kuz7micheva@glasnet.ru
Keywords: ternary chalcogenides, structure
types, coordination polihedra, stability regions
The binary to ternary compound transition - adding of a third component which can be IVIII group element as well as lanthanid ion with quite different characteristics may be performed either within the initial (corresponding to binary compound) structure or with formation of new structure types. In the latter case it can be assumed that changing of Ln-ion coordination encirclement is due to cation influence to each other, that partially imitates outer influences (such as pressure and temperature).
Possibility or impossibility of third component adding within an initial structure motive depends on three main factors: geometry (ionic radii ratio), energy (electronegativity ratio), and electronic configuration factor of lattice sharing cations. According to this considerations the "similarity" evaluation criterion was developed:
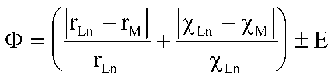 ,
,
where c means electronegativity, r - cation radius, and A - constant that accounts for electronic configuration pecularities of a cation. Figure shows that growth of F criterion correspondes to reducing of Ln and M ions "similarity". According to the developed criterion and using available structure data we calculated "stability regions" for different structure types. All compounds were divided into subgroups:
Analysis of these "stability regions" has helped to allocate different types of derived structures: polytypes, polysomes, superstructures and deficient phases and also to reveal following rules:
Developed stability fields are to be used for structure
prediction of new phases and evaluation of structure refinement
correctness.