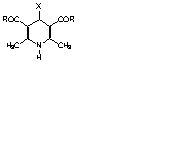
STRUCTURAL STUDIES OF 1,4-DIHYDROPYRIDINES DERIVATIVES
Héctor Novoa de Armas 1,2, Norbert Blaton 2, Maurice O. Peeters 2, Camiel De Ranter 2, Margarita Suárez Navarro 3, Emilio Rolando Colón 3, Yamila Verdecia Reyes 3, Estael Ochoa Rodríguez 3
1 Center of Pharmaceutical
Chemistry. P O Box 16042. Havana, Cuba.
2 Katholieke Universiteit Leuven. Laboratorium voor Analytische
Chemie en Medicinale Fysicochemie, Faculteit Farmaceutische
Wetenschappen, Van Evensstraat 4, B- 3000 Leuven, Belgium.
3 Organic Synthesis Laboratory.
University of Havana. P O Box 10400. Havana, Cuba.
Keywords: 1,4-dihydropyridines; calcium
channel antagonists; X-ray analysis; molecular conformation;
crystal structure.
1,4-Dihydropyridines (1,4 DHP) such as nifedipine and other related structures are the most important calcium antagonists and are well established drugs for the treatment of cardiovascular disorders such as angina and hypertension [1, 2]. Calcium antagonistic activity of members of the 1,4 DHP family is influenced by (a) the presence of the 1,4 DHP moiety, (b) alkyl groups (preferably methyl) substituted at the 2 and 6 positions, (c) ester groups at the 3 and 5 positions, (d) an aromatic (phenyl) substituent at the position 4 and (e) an H atom on N1 [1, 3-5]. Structure-activity studies have demonstrated that flattering of the boat conformation of the 1,4 DHP ring with the N atom at the prow and the aromatic ring in pseudoaxial position, correlates with increased activity, presumably due to the concurrent change in the position of the aromatic ring [1, 6]. The effect of the size of the substituent (R) in the side chain on the conformation is poorly understood: an increase in the bulk of the substituent groups might lead to greater perpendicularity of the dual ring system in 1,4 DHP compounds [7, 8]. The crystal structure analyses of a series of 1,4 DHP compounds were carried out in order to investigate their conformational features and to evaluate structural differences or similarities among these compounds and to attempt to relate these differences to therapeutic potencies.

| R | X | |
| I | CH3 | p-OH-Ph |
| II | CH3 | p-N(CH3)2-Ph |
| III | CH3 | Pyr |
| IV | OCH3 | Pyr |
| V | OCH2CH3 | Pyr |