2International Tomography Center, Sib. Branch of RAS, Institutskaya St., 3a, 630090 Novisibirsk, Russia
3Institute of Inorganic Chemistry, Sib. Br. of RAS, Akad Lavrentiev Av., 7, 630090 Novosibirsk, Russia
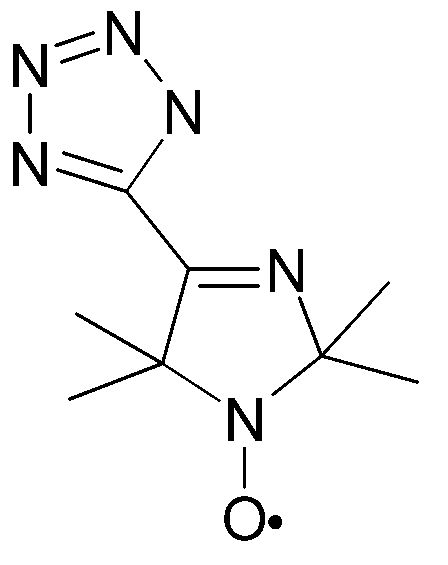
CRYSTAL STRUCTURE OF COPPER(II) COMPLEX WITH 4-TETRAZOLYL-3-IMIDAZOLINE-1-OXYL NITROXIDE
V.A. Reznikov1, A.B. Burdukov2, N.V. Pervukhina3, V.I. Ovcharenko2
1Institute
of Organic Chemistry, Sib. Branch of RAS, Akad Lavrentiev Av., 7, 630090
Novisibirsk, Russia
2International
Tomography Center, Sib. Branch of RAS, Institutskaya St., 3a,
630090 Novisibirsk, Russia
3Institute of Inorganic Chemistry, Sib. Br. of
RAS, Akad Lavrentiev Av., 7,
630090 Novosibirsk, Russia

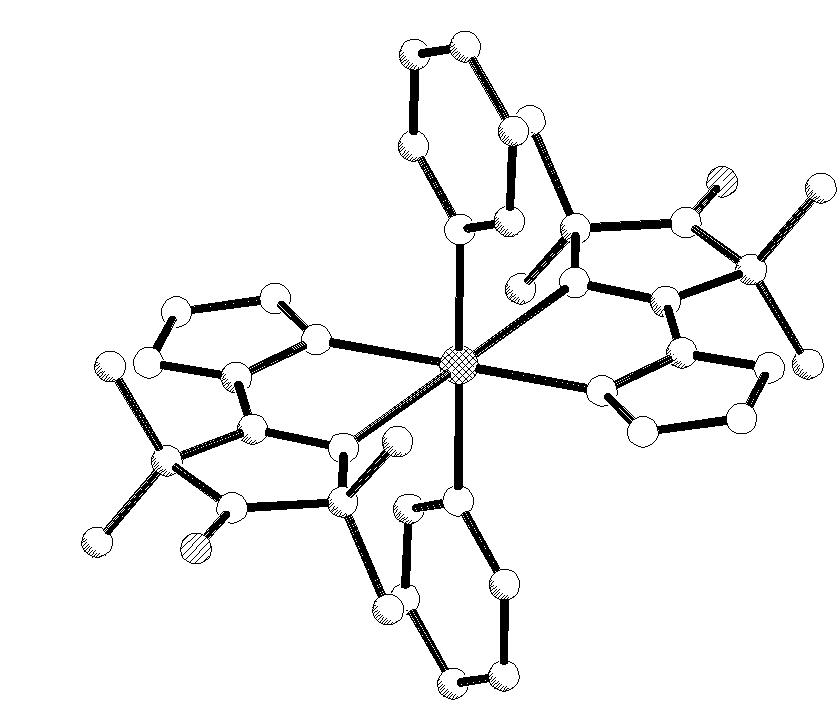
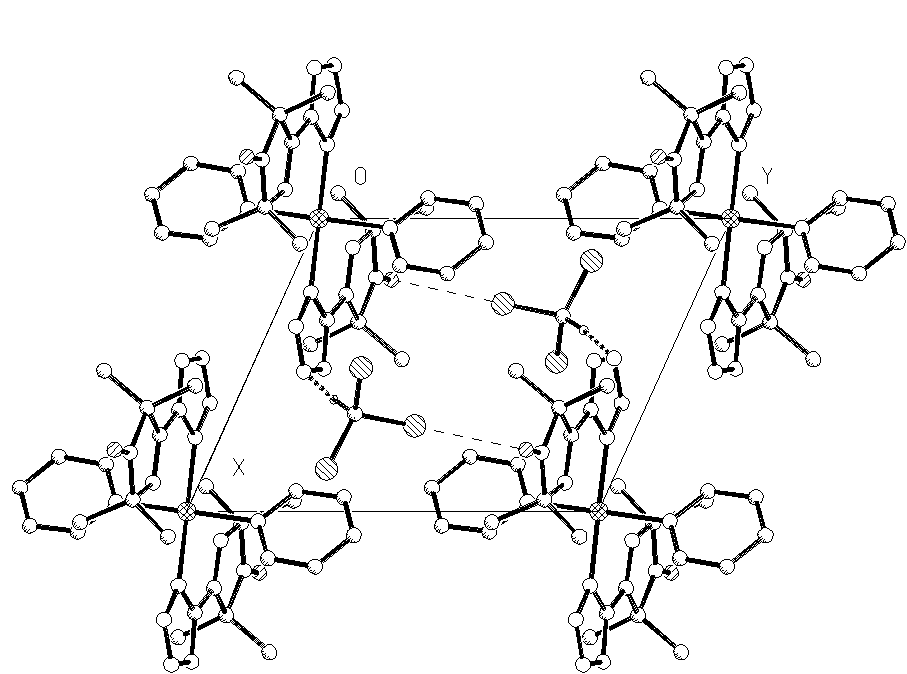
A copper complex of 4-tetrazolyl-3-imidazoline-1-oxyl nitroxide (L) has been synthesized and isolated as CuL2Py2.CHCl3. (triclinic, sp.gr. P(-1), a=8.611(2), b=11.182(2), c=12.169(2) A, a =102.88(1), b =98.60(1), g =111.127(1) deg, Z=2, 2262 Ihkl >2/304 params, R=0.046) The copper atom is hexacoordinated with four nitrogens of two nitroxides and two nitrogens of two pyridines. It manifests a pronounced Jahn-Teller distortion with elongated copper-imine nitrogen bonds (2.562(3) A), the lengthening of this chelate bond beeing observed for the first time among copper - 3-imidazoline nitroxide chelates. The compound incorporates chloroform molecules which fill the channels running along the c axis. Each chloroform molecule makes a short Cl-O contact (3.114 A) with the nitroxide oxygen, and the chloroform hydrogen weakly interacts with the tetrazole ring (H-N 2.48 A).
 |
 |