DO SUBSTITUTED TETRAHYDROFURANS ADOPT MINIMUM CONFORMERS IN THE SOLID STATE?
J. Hartung1, I. Svoboda2, H. Fuess2
1 Institut für Organische
Chemie der Universtität Würzburg, Am Hubland, D-97074
Würzburg, Germany
2 Fachbereich Materialwissenschaft,
Fachgebiet Strukturforschung, Technische Universität Darmstadt,
Petersenstraße 23, D-64287 Darmstadt, Germany
Keywords: Organic Compounds, X-ray analysis, Conformational analysis, Tetrahydrofuran, Computational chemistry
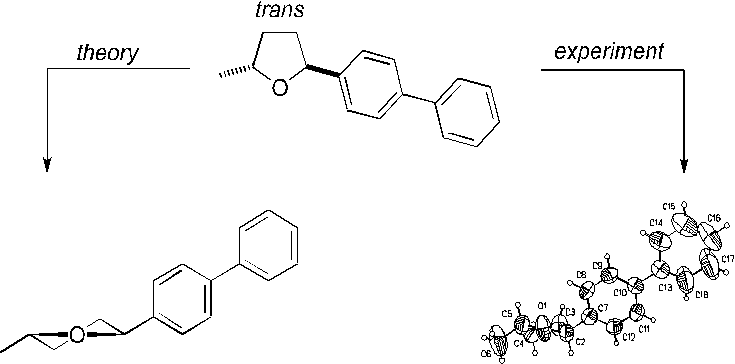
X-Ray crystallography and computational methods are powerful tools for conformational analyses of cyclic organic compounds. Whereas numerous studies on six-membered rings have been performed, substitutent effects on conformational behaviour of cyclopentanes and of tetrahydrofurans are far from being fully understood [1]. Substituted tetrahydrofurans however occur widely in nature and many derivatives thereof exhibit fundamental physiological effects. A detailed knowledge of substituent effects in tetrahydrofuran should therefore be of interest for both synthetic and medicinal chemistry. In order to accomplish this achievement, we have synthesized and crystallized disubstituted tetrahydrofurans, and have investigated the new compounds by X-ray diffraction [2]. In addition, calculations (force field, ab initio RHF/6-31G*) were carried out on a number of selected molecules [3].
Our results showed that tetrahydrofuran moieties of almost all structures adopted a conformation which would have been predicted according to our theoretical considerations. In two cases, solid state geometries of the central five-membered ring did not reflect calculated global minima. However, these structures were only offset by less than 1 kcal mol-1 in relative energy from computed minimum comformations in the gas phase.

Full details on structural investigations of disubstituted tetrahydrofurans and a short condensation of our guidelines for rationalization of substituent effects in tetrahydrofurans will be given in this contribution.
[1] E.L. Eliel, S.H. Wilen, Stereochemistry of Organic
Compounds, Wiley, New York, 1994.
[2] J. Hartung, R. Kneuer, I. Svoboda, H. Fuess, manuscript in
preparation.
[3] J. Hartung, manuscript in preparation.