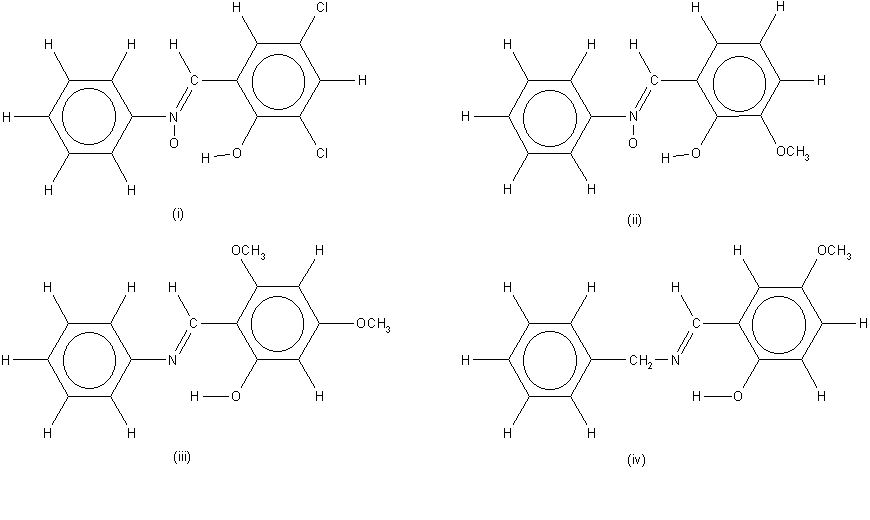
CRYSTAL AND MOLECULAR STRUCTURES OF SCHIFF BASES AND THEIR DERIVATIVES
Beata Stepien, Romana Anulewicz and Tadeusz Marek Krygowski
Department of Chemistry, University of
Warsaw, Pasteura 1, 02-093 Warsaw, Poland
E-mail: bestep@chem.uw.edu.pl
Crystal and molecular structures of
H-bonding of the above systems
is enriched by the data retrieved from CSD for the related
compounds. The main conclusions are as follows: The spacer
between the H-donating and H-accepting parts of the H-bond
exhibit a quite significant delocalization of p-electrons.
It is also observed a great differentiation of the degree of
delocalization measured by an application of HOMA index.1-3
This kind of effects may be related to an old idea of
quasiaromaticity. No relation between quasiaromaticity of the
spacer and the aromatic character of the ring partly contributing
to the spacer was found.
