STRUCTURE INVESTIGATION OF TWO NK-1 TACHYKININ RECEPTOR ANTAGONISTS
A. Ettorre1, C. Fincham1, R. Terracciano1, C. Toniolo2 and M. Crisma2
1Chemistry
Department, Menarini Ricerche S.p.A., 00040 Pomezia (Rome),
Italy,
e-mail: alex@isc.mlib.cnr.it
2Biopolymer Research Centre, C.N.R.,
Department of Organic Chemistry, University of Padova, 35131
Padova, Italy
Keywords: tachykinin antagonists, substance P
antagonists, X-ray structure
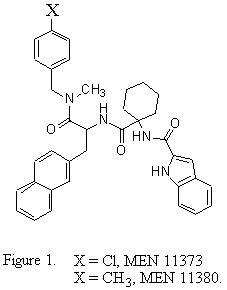
Substance P (SP), an endogenous undecapeptide belonging to the tachykinin family, is the natural ligand for the neurokinin 1 (NK-1) receptor. SP receptor antagonists have been investigated for their potential pharmacological application in inflammatory diseases [1]. In their class of compounds [2] MEN 11373 and MEN 11380 (figure 1) showed the most interesting biological activity.

The torsion angle values of the peptide backbone for the
(2)-naphthylalanine and cycloaliphatic residues are typical of a b-sheet and a
left-handed helical conformation, respectively. In both peptides the cyclohexyl ring adopts a
chair conformation with the carbonyl group in the
equatorial position. The N-methylamide group is in the
Z-conformation, and the torsion angle between the carbonyl and
indolyl groups shows a deviation from planarity of 12.3°(3) (MEN
11380) and 21.4°(2) (MEN 11373). In the crystal of MEN 11380 a water molecule forms a hydrogen bond bridge
between two symmetry-related peptide molecules. The
naphthyl and phenyl residues of MEN 11373 prefer an
intramolecular edge-to-face stacking, while in MEN 11380 they are
involved in intermolecular van der Waals
interactions.
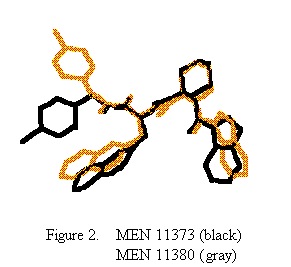
The overlap of the two structures (figure 2) well illustrates the overall similarity of the peptide backbone. On the basis of these two structures, together with the results of previous detailed NMR investigations on these and other active and inactive analogs, we argue that the most important feature in determining the biological activity is the position of the indolyl moiety. Indeed, as the Ca atom of the cyclohexyl moiety is pro-chiral, the left- and right-handed helical conformations are both accessible to this residue [3]. As a direct consequence, the indolyl moiety may occupy two very distinct space regions, leading to a dramatic effect on the overall structure of the molecule and, as a result, on the interaction with the protein receptor. Further structural investigations are currently under way to confirm the relationship between the indole position and the antagonist properties of the peptide.
1. C.A.Maggi, R.Patacchini, P.Rovero, A.Giachetti, J. Auton. Pharmacol. 13 (1993), 23-28.
2. S.Sisto et al.: Patent WO 95/19966, 27 July 1995.
3. C.Toniolo, Janssen Chim. Acta, 11 (1993), 10-16.