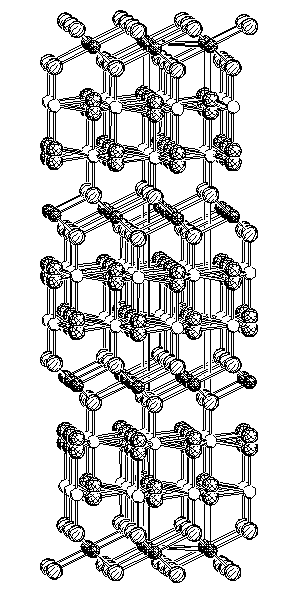
Figure 1: Crystal structure of Al3,89C2,66N0,34
Figure 2: FT-IR
(above) and FT Raman-spectra (below) of Al4C3
SYNTHESIS AND CRYSTAL STRUCTURES OF TERNARY PHASES IN THE SYSTEM Al/C/N
F. D. Meyer1, H. Hillebrecht2
1
Institut für Anorganische und Analytische Chemie und
Materialforschungszentrum FMF, Albert-Ludwigs-Universität,
Albertstr. 21, D - 79104 Freiburg, Germany
2 Institut für Anorganische Chemie, Universität
Bonn, Gerhard-Domagk-Str.1, D-53121 Bonn, Germany
Keywords: Crystal structure,
Al5C3N, blue AlN, Al4-x/3C3-xNx, vibrational spectra,
nitrides, carbides
Ternary phases in the system Al/C/N are well known [1,2]. They are refractories and posssible high-temperature semiconductors.
Single crystals of Al5C3N could be grown by high temperature reaction out of the gas phase. In contrary to Jeffrey and Wu [1,2] our single crystal structure analysis showed that the structure is centrosymmetrical and consists of Al3C2N- and Al2C-layers.
Furthermore we found a new phase, an N-containing aluminium carbide with a variable amount of nitrogen. The substitution of carbon by nitrogen in Al4C3 was already described [3], but in a following publication of the same authors, the nitrogen-content was withdrawn [4]. Nitrogen substitutes partly the [4+1] coordinated C2-atom. The Al1-position is not fully occupied, so that the formula Al4-x/3C3-xNx with x = 00,34 results. Al4-x/3C3-xNx is isotypic to Al4C3 and crystallizes in SG R3_m (fig. 1) but the cell constants are significant smaller (Al4C3: a = 3.3377(1) A, c = 24.9889(13) A, Al3.89C2.66N0.34: a = 3.3175(11) A, c = 24.842(7) A).
One Al-position (Al2) in the crystal structure of Al3.89C2.66N0.34 is partly disordered. The results of the XRD single crystal structure analysis are confirmed by quantitative analysis by the means of WDX investigations. Furthermore the UV/Vis spectra and the vibrational spectra of Al4C3 (fig. 2) and Al3.89C2.66N0.34 (fig. 3) are different.
During our preparative investigations in the system Al/C/N we found blue, transparent, hexagonal platelets with maximum size of 1.5 mm diameter. The crystals grew mostly on the lid of the graphite crucible, it seems that they were built out of the gas phase. With a single crystal structure analysis it could be shown that these crystals are AlN. The lattice constants of blue AlN (a = 3.1183(2) A, c = 4.9886(4) A) are enlarged compared to colourless AlN (a = 3.1132(2) A, c = 4.9829(3) A). Blue AlN has been described before in literature [5,6], but it wasn't very good characterized. The UV/Vis spectra of blue AlN shows a broad absorption peak at the region of 600-650 nm (fig. 4) which is in good agreement with the blue colour.
Some authors explain the blue colour by carbon
or oxygen contamination [7]. Despite careful quantitative
analysis of the blue crystals by the means of WDX investigations,
we couldn't find any carbon, but a small amout of oxygen.
 |
|
Figure 1: Crystal structure of Al3,89C2,66N0,34 |
Figure 2: FT-IR
(above) and FT Raman-spectra (below) of Al4C3 |
| Figure 3: FT-IR (above) and FT-Raman spectra (below) of Al3,89C2,66N0,34 | Figure 4: UV/Vis spectra of
colourless (above) and blue (below) AlN. |