MODULAR ASPECT IN THE STRUCTURES OF CUPRATES
V.Maltsev1, L.Leonyuk1, G.-J.Babonas2, D.Pushcharovsky1
1 Chair of Crystallography, Department of
Geology, Moscow State University, 119899 Moscow, Russia
2 Semiconductor Physics Institute, Gostauto
11, LT-2600 Vilnius, Lithuania
Keywords: cuprates, structures, modular
aspect
The principles of a modern structural classification of minerals were defined in [1,2] basing on the analysis of the bond character and strength. Using these principles the class of cuprates was distinguished among the copper compounds and their crystal-chemistry classification was proposed [3]. In the present report the modular aspects in the structures of cuprates were analyzed and the polysomatic series were characterised.
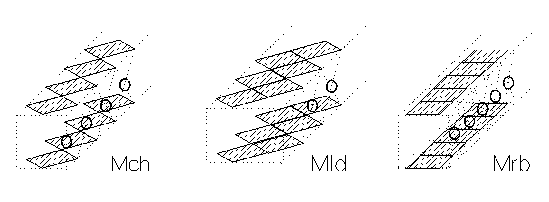
In the modular approximation the main units in the structure of cuprates are 1D modules of chains (Mch), ribbons (Mrb) and ladders (Mld) in which the Cu-O squares share corners, edges and both corners and edges, respectively, and are combined with cuprate-forming cations M (Fig. 1) [3]. The 1D modules Mch and Mld are relatively isolated in the structures of Ca2CuO3 and SrCuO2, respectively. The 1D modules are polymerized forming the 2D modules consisting of ordinary CuO2 and ladder-type Cu2O3 planes in CaCuO2 (infinite layer (IL) structure) and SrCu2O3, respectively, combined with corresponding cuprate-forming cations.
Using the modular approximation the polysomatic series can be
distinguished [4] in the family of cuprates. For example, the (M2Cu2O3)m(CuO2)n
compounds with the ladder-type structure can be considered. The
compounds are represented as the polysomes AB in the polysomatic
series with the end-members Sr0.73CuO2 [5]
(A) (structural type LiCuO2) and SrCu2O3
(B) of the ladder-type structure containing the planes of the
polymerized Cu-O ribbons.
The modular approximation was shown to be useful for the
interpretation of the structure and physical properties of
cuprates. For example, the structure of Bi-2212-type crystals was
analyzed. Two types of the Bi-2212-phases were distinguished
considering the structure of the Bi2O2-fragment.
If the Bi2O2-fragment possesses the
rock-salt (RS) type structure, the Bi-2212-phase represents the
member of polytypic series composed of the fragments Bi2O2
(RS)+SrO (RS)+CaCuO2 (IL). In this case the modulation
of lattice parameters typical for Bi-2212 compounds is absent. If
the Bi2O2-fragment retains the structure of
Bi-oxide, the Bi-2212-phase is a polysome AB in the series with
end-members Sr2CaCu2O6 (A) and
Bi2O3-x (B). In the latter case the
different symmetry of the slabs A and B leads to the modulation
of the lattice parameters. Superconducting samples were found
among the phases of both types. However, anomaly high Tc-values
were indicated in the phases of the second type. The solid-phase
reaction noted has lead to the formation of a new phase of
orthorhombic F-symmetry with lattice parameters a=3.826(3)
A, b= 3.823 (4) A, c= 15.29(1) Aand Tc=43
K.

Fig. 1. The 1D modules of chains
(Mch), ribbons (Mrb) and ladders (Mld) in the structures of
cuprates
The modular approximation was also applied to analyze the structure of SrCuO2. From this point of view, the structure of SrCuO2 can be considered as consisting of one-type units, the composition of which can be slightly different in the crystal.
A structural metastability is typical of such a complex compound with a possible manifestation of superconductivity [6]. The superconductivity (Tc=80 K) was indicated in (Sr,Ca)1-xCuO2 crystals with a large deviation up to 3 at % from a stoichiometric composition.
The modular aspects of the structure and the physical
properties were analyzed also in the other complex cuprates.
[1] J.Lima-de-Faria et al.: Acta Cryst. A
46 (1990) 1.
[2] J.Lima-de-Faria: Structural Mineralogy (1994).
[3] L.Leonyuk et al.: Kristallografiya 43 (1998)
291.
[4]. L.Leonyuk et al.: J. Mod. Phys. B 00 (1998)
000.
[5] J.Karpinski et al.: Physica C 274 (1997) 99.
[6] B.T.Matthias: Physica 69 (1973) 54.