Yu.I. Vesnin
Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences 630090, Novosibirsk, prospekt Lavrent'eva, 3, Russia
Now a large body of experimental data has been accumulated whose analysis inevitably leads to the following conclusion. The atomic-molecular structure, while remaining a foundation of the crystal structure, is to be extended and supplemented by one more level of organization of crystalline matter - the secondary crystal structure. This conception is based on the notion of an "elementary unit" of a crystal (1970). This notion is equivalent to the notion of a "molecule" in chemistry. The elementary crystal unit is treated as an individual formation - a kind of a giant molecule of a solid. Based on this notion, a consistent theory of secondary crystal structure can be constructed [1,2]. Conclusions of this theory are in quantitative and qualitative agreement with experiment.
The main statements of the SCS theory [1,2].
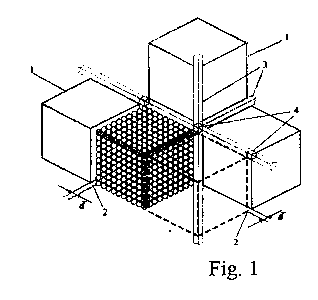
A single crystal consists of elementary units - mics (mic = minimal crystal). For a monoatomic crystal the typical mic size rM ~300Å . One mic contains nM = 106-108 atoms or molecules. The spaces between mics in a crystal form a single connected system, the T-space of the crystal, characterized by a decreased electron density and altered distances between the atoms. The elements of the T-space (Fig. 1, cubic crystal) are: a dol (2) (a 2-dimensional element), a lan (3) (1-dimensional), yam (4) (a node in the T-space). The basic parameter is the distance between the faces of adjacent mics d = 2-5Å for most of the inorganic crystal: d is equal to the interplanar distance for the most intense diffraction reflection. The elementary units (mics) unite to groups (blocks) which coalesce to form a single crystal.
The diffusion in a solid is treated by this theory as movement of impurity atoms through the T-space [2] . These atoms form a placement solid solution representing a separate type of solid-state solubility. There are three main types of placement solutions depending on impurity atom position in the T-space. The theory-based properties of such placement solutions are in good agreement with the known experimental data. The SCS theory quite adequately, in many case quantitatively, describes different experimental regularities that failed to receive a satisfactory explanation within the existing theories, such as the presence of two independent diffusion fluxes, the correlation between the diffusion coefficients of an impurity and its limiting solubility, the particular values of the limiting solubility of impurities, Guinier-Preston zone parameters and others.


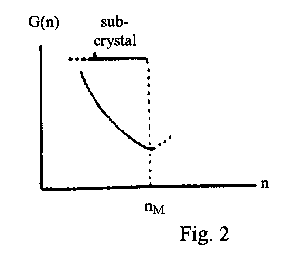
The main thermodynamic condition for elementary unit stability is the minimum on the size dependence of the free energy, Gr, at the crystal size r = rM. This is equivalent to the minimum in Gn when the number of structural units (atoms, molecules) n = nM (Fig. 2). At n < nM, the crystalline particle is a subcrystal which has an increased specific energy due to a deficit of mass relative to that of the mic and the associated increase in mutual potential energy of the structural units. As a consequence, such a subcrystal becomes a center of attractive forces for any surrounding atoms and molecules, i.e. a center of non-specific adsorption (an "active center"). This conclusion is of fundamental importance for the solid-state chemistry and materials science, the creation of active centers being the main way of reactivity control of solids. Such centers may be created by a mechanical treatment, surface etching, condensation of vapor and in other ways. It is the presence of a large number of subcrystals ("active centers") that is responsible for the unusual properties of the nano-materials (e.g. their content of energy). Subcrystals on a solid surface determine its adsorptive and catalytic properties.
The work [2, 5] reports some applications of the SCS theory to different fields of chemistry and physics of solids: atomic diffusion in a crystal, decomposition of solid solutions, heterogeneous catalysis mechanism, electronic transport in semiconductors (a negative resistance, Gunn effect). The application field of the SCS theory is constanly expanded.
Anomalies of the distribution coefficient and crystal properties over the region of small concentrations of isomorphous impurity.
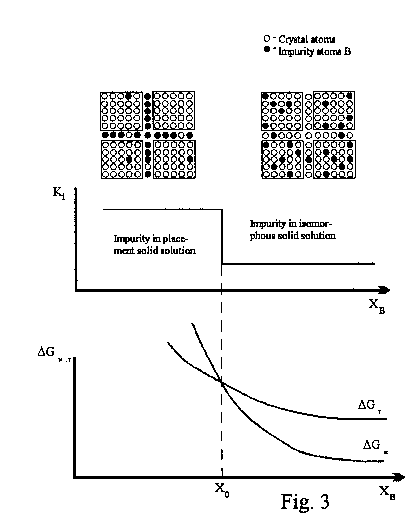
Anomalies of the distribution coefficient Ki on crystal growth from melt (the Ki jump at a small concentration XB of the isomorphous impurity) are of great importance in crystallization and purification processes. Such anomalies have been revealed for many binary system at XB < 2-3 at. or mol.% [3]. No commonly accepted explanation for this phenomenon exists. Anomalies of different semiconductive solid solution properties over the same concentration region (XB < 2 at.) have been found in [4].
Anomalies of different solid solution properties at small concentrations of the isomorphous impurity is one of the consequences of the secondary crystal structure theory [2]. According to this theory any single crystal consists of the elementary units (mycs) of size about 300Å . The intervals between mycs (25 for most inorganic crystals) form a unified system - T-space of the crystal that involves impurity atoms and proper crystal atoms. Those form a special class of solid solutions - placement solid solutions with the maximum concentration of 2-3 at. or mol. %.
A mechanism of entering an impurity into a crystal may be changed in the course of growing crystals at XB=XO < 2-3 %. At XB>XO, the impurity mainly occurs in the body of mycs as the isomorphous solid solution. At XB<XO, because of decreasing the enthropy factor and increasing free energy G, it is profitable for the impurity atoms to locate to interspacings of mycs forming a placement solid solution. A transition occurs in vicinity of the intersection point of the Gibbs mixing energy concentration curves Gmix=Hmix-TSmix. Figure shows the Gmix curves of isomorphous (GM) and placement solid solutions (GT). A prerequisite for such a transition is a sufficiently large positive value Hmix of the isomorphous solution. Otherwise, the GM curve is too low-lying without intersecting with the GT curve. Hmix attains a positive value due to the lattice deformation energy that is proportional to R (a difference in component atomic radii). Hence, the Ki jump is observed merely at a rather large R [3].
A change in the mechanism of entering an impurity into a crystal and in a solid solution type may result in an anomaly of different crystal properties [4] in the vicinity of XB=XO. The SCS theory allows us to estimate a character of crystal property changes with the Ki jump.