STRUCTURE DETERMINATION USING ELECTRON DIFFRACTION
Sven Hovm÷ller
Structural Chemistry, Stockholm University, S-106 91 Stockholm, Sweden
e-mail svenh@struc.su.se
Keywords: electron crystallography, electron
microscopy, electron diffraction, powder diffraction,
microcrystals, phase problem.
Electron crystallography is especially useful for structure determination of extremely small crystals, down to a few cubic nanometers, i.e. less than 100 unit cells. Electrons, unlike X-rays, can be focused into an image. This solves the phase problem of X-ray crystallography. With modern electron microscopes having a resolution of below 2 ┼, individual atoms can be seen and their positioned to an accuracy of about 0.2 ┼, which is sufficient for solving an unknown structure. Many crystal structures, ranging from inorganic compounds over organic molecules 1 to membrane proteins 2, have now been solved by electron microscopy.
For a refinement, phase information is not necessary, but on the other hand many high quality amplitudes are needed. Electron diffraction data extend to the same resolution as X-ray diffraction. Most inorganic crystals diffract beyond 1 ┼ resolution. A complete 3D data set can be obtained by tilting the crystal and taking diffraction patterns along different zone axes.
The two most severe problems of powder diffraction are overcome by electron diffraction; there is no problem of overlapping reflections, and the indexing of reflections is straight-forward. Recently, the direct methods program SIR97 has been designed to accept electron diffraction data, and several inorganic structures have now been solved in this way.
An example of an unknown inorganic crystal structure solved from electron microscopy images and refined against electron diffraction data is the metal cluster compound Ti11Se4 3. Attempts to grow single crystals suitable for X-ray diffraction and to solve the structure from X-ray powder diffraction had both failed. The crystal symmetry was found to be C2/m.

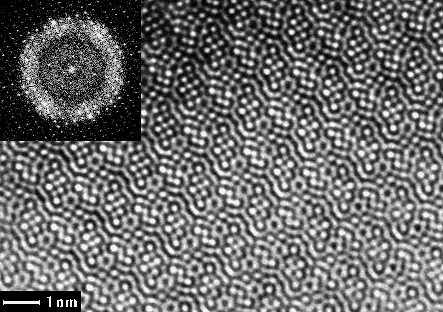
Fig 1. EM image of Ti11Se4 taken along the short b-axis and its calculated Fourier transform (FT) (inset). The lattice of diffraction spots was refined and amplitudes and phases extracted from this calculated FT.
Fig. 2. Electron diffraction
pattern of Ti11Se4.
The unit cell dimensions were a = 25.516, b = 3.4481, c = 19.201┼, = 117.84o (refined from X-ray powder diffraction). HRTEM images (Fig. 1) and electron diffraction patterns (Fig. 2) were taken, along the short b axis, on a 300 kV Philips CM30 electron microscope. The photographic negatives were digitised by a video-rate CCD camera. The 23 unique atoms were all found from one electron microscopy image with 1.75 ┼ resolution, after applying crystallographic image processing using the computer program system CRISP 4.
The atomic co-ordinates were refined to e.s.d. 0.02┼ against
electron diffraction (SAED) data to 0.75 ┼ resolution. There
were 113 unique reflections to 1.75 ┼ resolution in the Fourier
transform of the HRTEM images, whereas 408 reflections out to
0.75 ┼ could be quantified from the SAED patterns. The 23 atomic
co-ordinates (x and z) and temperature factors were refined
against all 408 reflections from SAED with SHELXL-93 5, but with
electron scattering factors for Ti and Se, giving a
crystallographic R-value of 14.7%. As can be seen in Fig. 3, the
structure of Ti11Se4 has great resemblances
with that of Ti8Se3 which has been solved
by single crystal X-ray diffraction 6. This renders further
evidence of the reliability of crystal structure determination
using electrons.
FIG. 3. Structure model of (left) Ti11Se4 obtained by electron crystallography and (right) the related structure Ti8Se3 solved by single crystal X-ray diffraction.
x= Ti6
octahedral clusters and = Se. The central layer of alternating
strings of two and four Ti octahedra parallel to the ab plane
is identical in the two structures.
Both electron crystallography and X-ray crystallography may gain from a combined approach, for example in the study of complicated crystal structures which form only very small crystals, powders, which are difficult to solve by X-ray diffraction and refine by the Rietveld method.
Although the scattering process of electrons and X-rays are
quite different - X-rays are scattered by the electrons while
electrons are scattered by the electrostatic potential - they
both give quite similar results, since both the electrons and the
potential are essentially point-like functions with high values
at the positions of the atoms and nearly zero in between. While
X-ray scattering increases linearly with atomic number, electron
scattering depends only on the net charge. Thus "heavy atoms
are not so heavy for electrons". One might expect then that
it would be easier to find lighter elements in the presence of
heavier ones, using electrons in stead of X-rays, for example
oxygen in metal oxides or even hydrogen atoms. Unfortunately, we
have not yet been able to confirm this experimentally.