
NEW NATURALLY OCCURRING PHASE H2[AsIII2O3(MoVIO2OH)2] . H2O OF SECONDARY ORIGIN FROM JACHYMOV (JOACHIMSTHAL)
Petr Ondrus1, Roman Skala1, Ivana Cisarova2 and Frantisek Veselovsky1
1 Czech
Geological Survey, Klarov 3, CZ-11821 Prague 1, Czech Republic,
2 Faculty of Science, Charles University, Hlavova
2030, CZ-12843 Prague 2, Czech Republic,
e-mail of correspondence author: ondrus@cgu.cz
Keywords: new mineral phase, crystal structure, Jáchymov
The mineral forms minute green to grey-green acicular crystals or continuous crusts, which rim strongly corroded veinlet. Crystal size varies between 0.1 to 0.5 mm. Crystals are lath shaped. Their termination is highly irregular with radiating parallelly organized needle-like features (Fig. 1). Laterally it gives way to grey-green scorodite in spherical and botryoidal aggregates. The phase was observed on fractures in proximity to strongly weathered arsenopyrite (löllingite)-pyrite vein approximately 5 cm thick, with the sulphides partly or completely altered to mixture of compact grey-black scorodite with a metallic lustre and arsenolite in the Geschieber vein, the Svornost shaft, Jáchymov ore district, Czech Republic. The phase was formed in highly acid environment of concentrated sulphuric acid, in the presence of As2O3 in significant concentrations. No mineral containing Mo was found in the assemblage. It is suggested that amorphous, finely dispersed Mo sulphides (jordisite) or oxides, soluble in the acid solutions, served as a source of Mo. The solutions carrying Mo6+ probably attained elevated concentrations which resulted in crystallisation of the new phase.
The quantitative chemical electron microprobe analysis: Mo 35.37, As 27.72, O 36.69 wt. % gives after recalculation based on 12 oxygen atoms the following empirical formula: 0.97 H2[As2O3(MoO2OH)2] . 1.35 H2O, which could be simplified to: H2[As2O3(MoO2OH)2] . H2O. For quantitative chemical analysis the following conditions were used: the standards: arsenolite (As, O), molybdenum (Mo). Operating voltage and sample current 20 kV and 20 nA respectively. Specimen beam size was 55 mm. Correction procedures ZAF, Phi(rhoZ), and Quadrilateral were used for calculation of all quantitative analyses.
Powder X-ray diffraction data give a = 7.0515(6), b = 12.0908(9), c = 12.2190(14) A, b = 101.268(9) °, V = 1021.7(2) A3. Measured density is 3.50(2) g.cm-3. The phase H2[As2O3(MoO2OH)2] . H2O is optically positive, X // b, Z/c = 12°, elongation negative, optical indices are a = 1.757( 2), b = 1.778 (2), g = 2.04 (1)o, birefrigence 0.28, 2Vcal = 35.12°, pleochroism is in X ~ Y light grey to light greenish grey, Z yellowish grey. Compatibility factor = -0.019.
The crystal structure was solved from single-crystal data. Basic motif of the structure is presented in Fig. 2. It consists of double-chains built up by two individual chains with a sequence ...O-As-O-Mo... interconnected by bridging oxygens and oxygen atoms belonging to a common apex of a bi-arsenite group. In interstitial spaces, there are water groups, probably comparable to channel or zeolitic water. Two structurally non-equivalent [MoO6] octahedra are slightly distorted as calculated from structure data by program VOLCAL [1]. Polyhedron around Mo1 is of larger volume and also its quadratic elongation and bond-angle variance have higher values indicating more severe distortion than in the case of the octahedron around Mo2.
Powder data were collected using Philips X'Pert diffractometer in range from 3 to 130.005 °2q CuKa with step 0.015 °2q CuKa and exposure of 10 sec. per step. Lattice dimensions were refined with program by Burnham [2] employing correction term (cosq.cotq)/l2 for sample displacement.
For the single crystal X-ray
measurement a needle-like fragment of the phase was mounted on a
glass fibre and measured using the four-circle diffractometer
CAD4-MACHIII with MoKa radiation.
The crystallographic data obtained are summarised in Tables. The
crystal structure was solved by direct methods (SHELXS86 [3]) and
refined by a full-matrix least-square procedure based on F2
(SHELXL93, [4]). Scattering factors were those employed in the
SHELX programs.
Crystal data for H2[As2O3(MoO2OH)2] . H2O
| Space group | P21/c |
| Unit cell dimensions: a [A] | 7.0398(4) |
| b [A] | 12.0682(13) |
| c [A] | 12.210(2) |
| b [°] | 101.265(9) |
| V [A3] | 1017.4(2) |
| Z | 4 |
| Density (calculated) [g.cm-3] | 3.524 |
| Absorption coefficient [mm-1] | 8.98 |
| F(000) | 1008 |
| Crystal size [mm3] | 0.10 0.14 0.39 |
| Theta range for data collection [°Q] | 2.40 to 24.99 |
| Index ranges : h | 0; 8 |
| k | 0; 14 |
| l | -14; 14 |
| Reflections collected | 1935 |
| Independent reflections | 1787 [R(int) = 0.0489] |
| Refinement method | Full-matrix LSQ on F2 |
| Data / restraints / parameters | 1786 / 0 / 145 |
| GOF | 1.07 |
| Final R indices [I>2s(I)] | R1 = 0.0455, wR2 = 0.1143 |
| R indices (all data) | R1 = 0.0580, wR2 = 0.1243 |
| D/s (max) | 1.745 |
| D(r) [e.A-3] | -1.728 |
| R1 = S||Fo
|- |Fc |/ S |Fo | wR2 = {S[w(Fo2-Fc2)2] / S[w(Fo2)2]}0.5 GOF = {S[w(Fo2-Fc2)2]/(N - P)}0.5 |
|
Atomic coordinates ( 104) and equivalent isotropic displacement parameters [A2 103] for H2[As2O3(MoO2OH)2] . H2O. Ueq is defined as one third of the trace of the orthogonalized Uij tensor.
| x | y | z | Ueq | |
| Mo(1) | 6214(1) | 2121(1) | 2757(1) | 12(1) |
| Mo(2) | 1799(1) | 2321(1) | 905(1) | 13(1) |
| As(3) | 2066(1) | 1418(1) | -1626(1) | 13(1) |
| As(4) | 6245(1) | 3440(1) | 5030(1) | 13(1) |
| O(1) | -333(9) | 2915(5) | 1068(5) | 24(2) |
| O(2) | 4963(8) | 1976(5) | 1095(4) | 14(1) |
| O(3) | 1387(10) | 951(5) | 1006(5) | 26(2) |
| O(4) | 8084(9) | 1267(5) | 2671(5) | 22(1) |
| O(5) | 7186(9) | 3414(5) | 2745(5) | 22(1) |
| O(6) | 3147(8) | 2659(5) | 2485(4) | 14(1) |
| O(7) | 1681(9) | 2432(5) | -668(5) | 19(1) |
| O(8) | 4275(8) | 840(5) | -875(4) | 14(1) |
| O(9) | 6029(9) | 2158(5) | 4300(5) | 18(1) |
| O(10) | 4588(9) | 470(5) | 2832(5) | 22(1) |
| O(11) | 2886(11) | 4090(6) | 742(6) | 34(2) |
| O(12W) | 1477(9) | 137(6) | 3705(5) | 25(2) |

Fig. 1. Aggregate of acicular crystals of the phase H2[As2O3(MoO2OH)2] . H2O. Note crystals termination, which is highly irregular with radiating parallelly organized needle-like features.
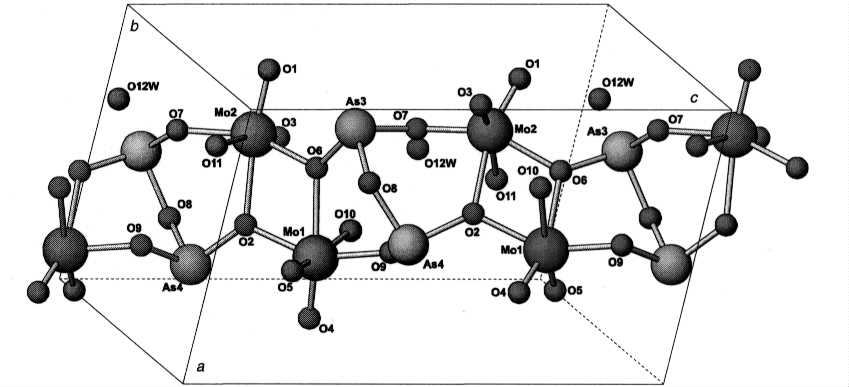
Fig. 2. Basic structure
motif found in crystal structure of H2[As2O3(MoO2OH)2]
. H2O
X-ray powder diffraction pattern of [(MoO2)2As2O5(H2O)2] . H2O from Jáchymov.