MICROSTRUCTURAL FEATURES OF NATURAL VEIN QUARTZ
Dilyara B. Gubareva, N.G. Stenina
United Institute of Geology, Academician Koptyug pr. 3, 630090, Novosibirsk, Russia, stenina@uiggm.nsc.ru
Keywords: microstructure of natural silicium dioxide, ore capacity of vein quartz
Being one of the most main mineral of the Earth Crust, silicium dioxide and its numerous varieties are of great importance in fundamental and applied aspects of material sciences. Despite SiO2 have being thoroughly investigated the keen question of the water speciation in quartz is under discussion till nowadays. The aim of this paper is to determine microstructural and crystallochemical features of gold-bearing vein quartz in terms of the concept of aquacomplex (1). The concept fits a problem of quartz ore-bearing capacity to the one concerning water speciation in silica matrix. Quartz from the vein with gold-sulfide mineralization and poor mineralized veins (Sarala ore field, Kuznetskii Alatau, Russia) (2) was studied by the complex of methods: TEM as a method for resolving of quartz lattice defects, complemented by IRS, X-ray diffractometry, thermal and chemical analyses.
Taking into account that
the terms "ore-bearing" and "non ore-bearing"
relative to vein quartz mainly economical significance, chemical
analysis (AAS on SP-9) was employed to determine trace elements
in silica matrix. These data are given in Table 1. Assuming these
results the first quartz was classified as
"ore-bearing" (I) and the second - as "non
ore-bearing"(II).
Table 1. Trace elements concentrations in quartz (g/t).
| Quartz | Au | Ag | Fe | Al | Na | Li |
| I | 5.23 | 4.29 | 1660 | 1820 | 146 | 0.88 |
| II | 0.04 | 0.18 | 900 | 1620 | 52 | 0.3 |
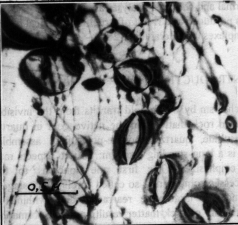
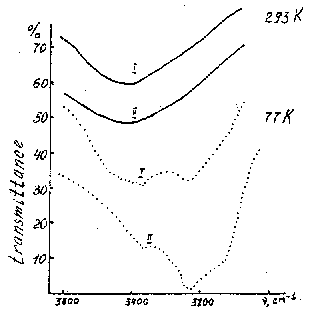
TEM and IRS showed principal differences in the water state in these contrast quartz samples. In the ore-bearing quartz water is incorporated into submicron gel-like defects, so-called "voids" (fig. 1), which have been interpreted as clusters of aquacomplexes; single aquacomplexes enter also crystallographically into quartz lattice. IRS evidenced that this water is structurally and chemically bonded in silica matrix. Broad isotropic peak centered at 3450 cm-1, which characterizes molecular water in unordered silica environment becomes only broader at the liquid nitrogen temperature whereas this peak in non ore-bearing quartz displaces completely to the ice band at 3200 cm-1 under 77 K (fig.2).
Fig. 1


Fig. 2
According to the TEM data the second quartz has a large concentration of submicron water containing bubbles, i.e. it contains water in a free form. Thermal analysis presented qualitative and quantitative data on the water state in both types of quartz (fig. 3).
Fig. 3

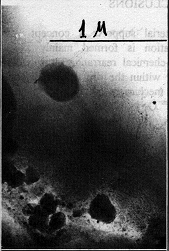 Fig. 4
Fig. 4
Water content in the ore bearing quartz is 2.5 more than in non ore-bearing one, temperatures of its escape from the silica bulk are 650oC and 350, 450oC correspondingly. X-ray diffraction revealed that a lattice of ore-bearing quartz is more strained in comparison with non ore-bearing one. TEM examination of ore-bearing samples showed that appearance of various quartz generations caused by structure-chemical transformations of silica lattice is connected with water and metal impurity redistribution and interaction. IRS data point that quartz closely associated with ore mineralization in comparison with quartz located near vein-host rock interface has elevated content of structurally bonded water. TEM showed that as ore minerals are approached, structure of vein quartz becomes more unordered; heterogeneous inclusions appear in the crystal bulk, such as cryptocrystalline phases and amorphous gel-like defects of one-some micron size. The last stage of these transformations may be named a an "ore birth". Fig. 4 illustrates this process. It can be seen that gel-like matter produces immediately ore phases.
Basing these data a model of aquacomplex defect in silica matrix was proposed.
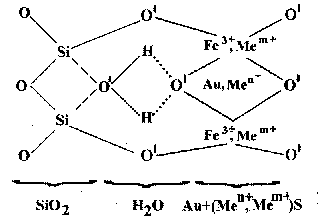
Fig. 5 Model of aquacomlex Men+- one-two valent metals; Mem+-polyvalent metals, non metals: P, B, As, O'- O, F, Cl; Si---O - donor-acceptor bond; H.....O - hydrogen bond.
In this model central H2O molecule which bonds left (SiO2) and right (Au2FeO) parts together by weak donor-acceptor and hydrogen bonds. This model explains such characteristics of H-related defects as: simultaneous behavior both as OH-group and H2O molecule, association with impurity defects (Na, Li, and Al), ability to form associations up to the «gel-like» defects. Quartz is the unique silicate mineral, which corresponds only to the left part of aquacomplex. The latter have a growth nature and express the structure of mineral-forming medium. Aquacomplexes fits into the framework only in quartz may represent point defects and their associations.
1. Stenina N. G. Geochimia, 5, 641-653, (1988) [
in Russian].
2. Stenina N. G., Gubareva
D. B. Mineral Deposits, 323-326, (1997).